Fig. 5.1
Atrial fibrillation with moderately fast ventricular response (about 100 bpm). RR intervals are irregular and coarse F waves are visible
European (ESC 2010) [4] and US (AHA/ACC/HRS 2014) [5] guidelines (GL) classify AF as first diagnosed or recurrent. Recurrent AF episodes are defined as paroxysmal if they terminate spontaneously (AHA/ACC/HRS 2014 GL [5] include also AF treated with cardioversion within 7 days), persistent (lasting longer than 7 days), long-lasting persistent (continuous for more than 12 months with intention of rhythm control strategy) or permanent (further attempts to restore and/or maintain sinus rhythm excluded).
AF may be asymptomatic, so the patient may be referred to the ED by a general physician for an occasional finding of irregular heart rhythm, or symptomatic. Symptoms described on arrival at the ED are most frequently palpitations (69.2 %), followed by dyspnoea (27.5 %), congestive heart failure (10.9 %), fatigue (105), angina (9 %), pulmonary oedema (4.5 %), syncope (3.3 %), transitory ischaemic attack (TIA) or stroke (2.2 %) [2]. Apart from acute symptoms, atrial fibrillation causes a fivefold risk of stroke, a threefold risk of heart failure and doubles dementia and mortality [5]. Furthermore, it reduces the quality of life [6]. For stroke, risk stratification and treatment of nonvalvular and valvular AF are distinguished. Valvular AF carries a very high thromboembolic risk if not anticoagulated: in patients with mitral stenosis and AF it is about 20 times over patients not affected by AF. Definition of valvular AF is not univocal; according to the AHA/ACC/HRS 2014 Guidelines, it is defined as AF associated with rheumatic mitral stenosis, a mechanical or bioprosthetic heart valve or mitral valve repair [5].
Atrial flutter (AFL) is less common (about 10 % of ED admissions for AF or AFL were due to AFL in the “FIRE” registry [2]) but shares similar clinical features and treatment with AF. It is a macroreentrant atrial arrhythmia with regular atrial activation and shows electrocardiographically continuous atrial activity in at least one lead which may have various morphologies depending on the location of the circuit. The ventricular rate is usually regular due to a fixed atrioventricular (AV) conduction rate, mostly 2:1 with a frequency of 120–150 bpm (Fig. 5.2), but may be slower or irregular due to a variable AV conduction rate.
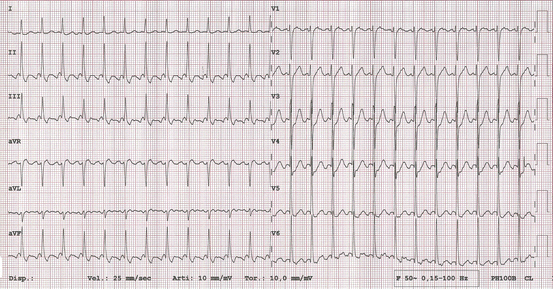

Fig. 5.2
Atrial flutter with 2:1 AV conduction and quite rapid ventricular rate of 150 bpm. Regular RR intervals
Both arrhythmias are usually associated with cardiovascular pathologies, mostly hypertension (64 %) but also coronary artery disease (32 %), heart failure (32 %), valvular hear disease (26 %) and cardiomyopathy (10 %). Chronic obstructive pulmonary disease (COPD) may be associated in 13 % of the patients and thyroid disease in 9 %. In about 10 %, none of these comorbidities are found, and the arrhythmia is called “lone atrial fibrillation” (or flutter), if the patient is younger than 65 years old [7].
Acute diseases may be associated with AF/AFL like myocardial infarction, pulmonary embolism, pneumonia, severe infections, alcohol abuse, drug toxicity, hypothermia and electrolyte abnormalities [8].
5.2 What Physicians Working in ED Should Know
The ED physician has several tasks:
1.
Rule out severe clinical instability
Altered mental status, hypotension, pulmonary oedema and ongoing ischemia necessitate acute electrical cardioversion (ECV) once diagnosis of AF/AFL is confirmed and is thought to be the primary cause [9], at least if there is no prompt response to rate control therapy [4, 5, 10].
2.
Diagnosis of the arrhythmia
The pulse presents irregular in case of AF but is usually regular in case of flutter. Electrocardiographic diagnosis of AF is usually easy for the irregular RR intervals and f waves. Atrial flutter appears usually as a regular arrhythmia due to fixed conduction rate, mostly 2:1 with ventricular rates around 120–150 bpm, but may be less or conduction may be variable. When rapid conduction is present, the flutter waves may not be so evident. In these cases and if the patient is stable, we recommend to perform a continuous 12-lead ECG registration during carotid sinus massage (after exclusion of any carotid bruit) or adenosine infusion (6, 12 or 18 mg bolus followed by 20 ml saline solution flush) to clear up differential diagnosis with other supraventricular arrhythmias and to record the flutter wave morphology, which helps the cardiologist to define the long-term therapeutic approach (e.g. radiofrequency ablation). Typical atrial flutter (also called cavotricuspid isthmus-dependent atrial flutter) is a macroreentrant circuit posterior to the tricuspid annulus, crossing the isthmus between the inferior vena cava orifice and tricuspid annulus. Activation sequence is more frequently counterclockwise (downwards the right atrial free wall and upwards the interatrial septum), and in this case, the arrhythmia is called common atrial flutter or counterclockwise AFL (Fig. 5.3). Less common is clockwise typical atrial flutter, also called reverse AFL (Fig. 5.4). On ECG, the former shows classical “sawtooth” flutter waves with negative polarity in inferior leads and positive in V1, and the latter shows flutter waves with positive polarity in the inferior leads and negative in V1. Atypical flutters (noncavotricuspid isthmus dependent) show flutter waves with other morphologies (Fig. 5.5) [5].
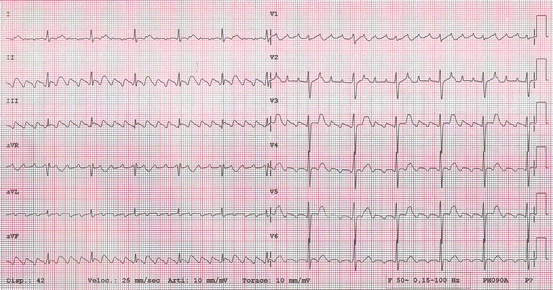
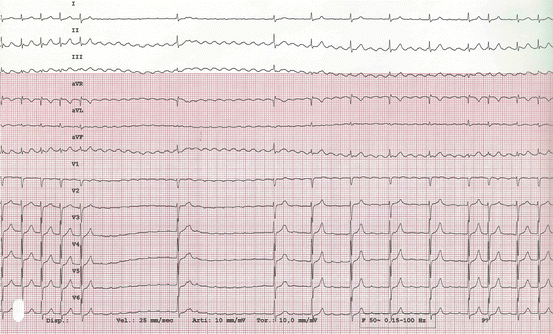
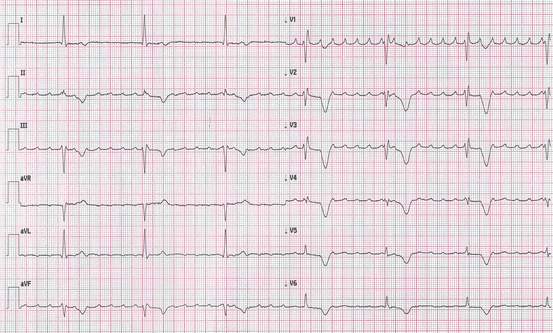

Fig. 5.3
Typical atrial flutter with counterclockwise atrial activation (common atrial flutter) with 4:1 AV conduction, 70 bpm. Atrial flutter waves are clearly seen: negative in inferior leads and positive in V1

Fig. 5.4
Typical atrial flutter with clockwise atrial activation (reverse atrial flutter) during adenosine injection. Initially 2:1 AV conduction, 150 bpm and masked flutter waves. A marked conduction slowing follows, unmasking flutter waves: positive in inferior leads and negative in V1. Diagnosis was confirmed by electrophysiological study

Fig. 5.5
Atypical atrial flutter: flutter waves are positive in inferior leads and in V1
In patients with AFL treated with flecainide or propafenone sometimes rapid 1:1 AV conduction is favoured due to flutter wave slowing. This phenomenon is frequently accompained by conduction aberrancy and QRS widening (Fig. 5.6).
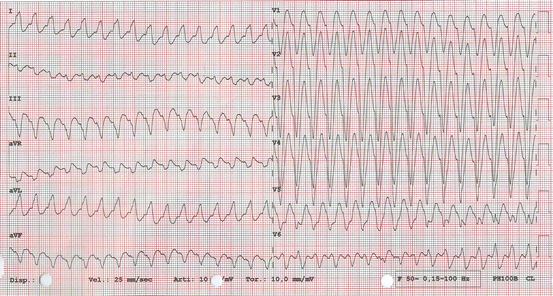

Fig. 5.6
Atrial flutter with 1:1 AV conduction and aberrancy 190 bpm (during i.v. flecainide infusion). Flutter waves are not visible. Resembles a wide QRS tachycardia
In patients with Wolff–Parkinson–White Syndrome, a pre-excited AF (Fig. 5.7) is characterised by irregular RR intervals with various degrees of QRS widening (various degrees of fusion between accessory pathway and nodal conduction). In case of fast conduction properties of the accessory pathway, ventricular rate can be extremely high, possibly leading to severe haemodynamic consequences or even to ventricular fibrillation. Pre-excited AFL appears as a wide QRS tachycardia.
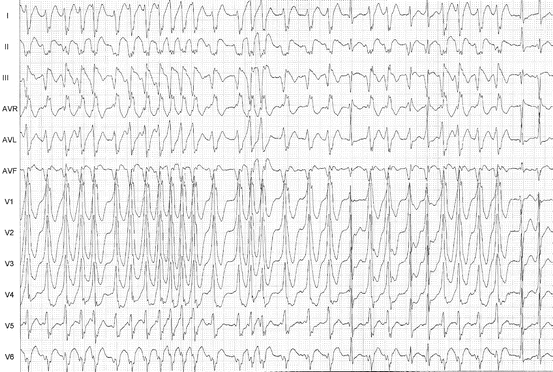

Fig. 5.7
Pre-excited atrial fibrillation with fast AV conduction, at times near 300 bpm. Bizarre wide QRS morphology with irregular RR intervals. In between, there are a few beats with normal QRS, conducted through the AV node
3.
Clinical history and physical examination for evaluation of concomitant heart disease and comorbidities
A complete medical history including previous cardiac and noncardiac diseases, history of former AF, medication use and drug or alcohol abuse should be obtained. Onset of the current AF episode should be carefully defined as it is a crucial point for decision making, in particular, it has to be cleared if onset is within or more than 48 h (time within acute cardioversion without previous anticoagulation can be considered). Frequently, AF presents as continuous palpitations with acute onset. In this case, onset definition is easy. Other times, palpitations are perceived intermittently or other symptoms dominate as dyspnoea or fatigue which can be vague. In these cases, it can be of help if patients are used to evaluate daily their pulse (often evaluated together with arterial pressure in the hypertensive). If onset of AF cannot be clearly dated, the arrhythmia should be regarded as “AF of undefined onset”.
Physical examination should initially be aimed to vital signs like blood pressure, pulse frequency, signs of pulmonary or peripheral congestion and oxygen saturation. Cardiac assessment should include evaluation of heart sounds and murmurs and a careful evaluation of both arterial and jugular pulse (to assess the rhythm and possible AV dissociation).
Laboratory tests should be tailored to patient’s presentation. A complete blood count, serum electrolytes, renal and hepatic function tests, coagulation status and thyroid-stimulating hormone should be routinely obtained. Brain natriuretic peptide, troponin I or T, C-reactive protein and D-dimer test help ruling out heart failure, myocardial ischemia, concomitant inflammatory or infectious disease and pulmonary embolism if these conditions are suspected.
Thyroid disease is not uncommon in AF patients and overt hyperthyroidism is associated in about 4 % of AF patients [11]. Thyroid-stimulating hormone is a reliable screening tool which can be applied in some EDs and may help in decision making (choosing rate control strategy or defining contraindication for amiodarone use [12].
An urgent echocardiogram should be performed in haemodynamically compromised patients [4]. Alternatively, a focused ultrasonography performed by the ED physician can determine underlying cardiac condition like structural heart disease, pericardial effusion and pulmonary embolism, or clarify causes of shock or hypotension and guide resuscitation by volume repletion measuring the inferior vena cava diameter. Ultrasound competence is not uniformly available among ED physicians, but a focused examination of the heart may help guiding therapy in AF management [13]. However, a complete echocardiographic examination should be performed as part of initial evaluation [4, 5, 14] as soon as possible, even on outpatient basis in stable patients.
4.
Symptom relief by cardioversion (electrical or pharmacological) or rate control
Unstable patients
Anticoagulation with low molecular weight heparin (LMWH) or unfractionated heparin (UFH) should be initiated as soon as possible. Do not delay ECV if the patient is extremely unstable [9]. In the author’s opinion if the patient is hypotensive, ECV is preferable, whereas if pulmonary oedema or myocardial ischaemia is present, rate control should be tried first.
Atrial fibrillation can be an epiphenomenon of an “alternative” primary diagnosis, and in these cases, mortality rate is high (11 % in the study of Atzema et al. [15]). Patients may present with vague symptoms. Scheuermeyer et al. [16] reported on a series of patients with underlying medical illness like sepsis, acute coronary syndrome (ACS), decompensated heart failure, pulmonary embolism, obstructive pulmonary disease exacerbation, acute renal failure and gastrointestinal bleedings. Those who underwent immediate rate or rhythm control attempts suffered a much higher complication rate (40.7 %) than those who did not (7.1 %) and were unlikely to achieve success. So it is more advisable to delay rhythm management strategies and manage these patients first correcting the primary acute disease, administrating intravenous fluids guided by bedside echocardiography (to assess volume status) and performing frequent reassessments while waiting for confirmatory diagnostic tests [16].
Stable patients with recent-onset AF (<48 h)
There is still controversy on optimal management of these patients, being rate and rhythm control the two alternative strategies without clear-cut evidence favouring one or the other. Rate control strategy in the ED consists of pharmacological control of ventricular rate and anticoagulation treatment if indicated. With rhythm control strategy, the patient is cardioverted to sinus rhythm (SR) either pharmacologically or electrically, usually discharged from the ED and followed as outpatient afterwards. A delayed cardioversion (CV) after at least 3 weeks of adequate anticoagulation can be planned in some patients if the initial rate control strategy is not well tolerated.
Although several trials on AF patients like the AFFIRM [17] did not find significant differences on the main clinical outcomes between rate and rhythm control, the latter seems the preferred strategy worldwide in the long treatment of AF [18, 19]. General indications for preference of either strategy are listed in Italian guidelines on AF management [20]: rhythm control strategy should be the option for (1) patients with first AF episode (taking into account also age and comorbidities) and (2) for patients with recurrent AF with high probability of long-term sinus rhythm maintenance, or in whom rate control proved uneffective or if AF determines negative haemodynamic effects. Rate control strategy is preferred for (1) patients refractory to antiarrhythmic therapy, with frequent AF relapses non-candidable to AF ablation or not candidable to rhythm control strategy because of age or underlying heart disease and (2) for older asymptomatic patients with persistent AF or older patients with recurrent AF, heart failure and poor ventricular function.
As far as ED practice concerns, there is a considerable variability among different countries: rate control strategy is largely favoured in the USA (74 %), while in Canada, most patients undergo cardioversion (66 %). The UK and Australia adopt rate or rhythm control in equal proportions [21].
In our clinic, rhythm control is generally the preferred management strategy.
The theoretical advantages of an aggressive rhythm control approach are rapid discharge without hospital admission, immediate resumption of normal activities, potential avoidance of medications and minimization of healthcare resources and costs. In addition, with rate control strategy, many patients can derive physical and psychological inconvenience maintaining the arrhythmia and drugs after discharge [22].
A recent review of five studies involving 1593 cases undergoing ED cardioversion [23] demonstrated that success rate was high (around 90 %), with a discharge rate of about 90 % and a complication rate generally lower than 5 %, exceptionally leading to hospital admission. Thromboembolic complication rate was very low (0.06 %). So in selected patients with recent-onset AF, ED physicians should feel comfortable with this approach which should ideally be standardised by a local ED protocol and include timely and appropriate follow-up. The first description of an ED protocol dealing with recent-onset AF or flutter was the “Ottawa Aggressive Protocol” [24]. Excluding patients presenting with AF onset >48 h or of unknown duration and those with other primary diagnosis necessitating admission, it involved sequential pharmacologic and, when indicated, electrical cardioversion by the emergency physician. It allowed discharge of 96.8 % of the 660 enrolled patients, 93.3 % of those in sinus rhythm, after a median length of stay of 4.9 h overall. Adverse outcomes, mainly transient hypotension, occurred in 7.6 % and only 3.2 % of patients required hospitalisation. No patient died or suffered a stroke. Despite these encouraging results, limited data help guide emergency physicians on which AF patients can be safely managed as outpatients (see below). An interesting implementation of an ED cardioversion protocol for acute AF patients after exclusion of high-risk features was described by Bellone et al. [13]; their workup included morphological echocardiographic investigation before treatment by certified emergency physicians, which has the potential of improving the risk stratification and the therapeutical choice.
Another management strategy consists in conservative “wait-and-watch approach” which relies on the known discrete likelihood of spontaneous cardioversion of recent-onset AF (from 20 % up to 68 % in the study of Danias et al. [25]); early (<24 h) presentation is the best predictor of spontaneous cardioversion. Patients may be discharged on rate control medication and anticoagulation if appropriate and scheduled for return visit to ED to permit CV within the 48 h period [26] from symptom onset if AF persists, or may be admitted to an ED observation unit, reassessed after some hours and eventually cardioverted if AF persists [27]. This strategy can be applied following the patient’s preference but may be an option if ED is overcrowded.
Stable patients with AF onset >48 h or with AF of uncertain onset
If the patient has properly been anticoagulated for at least the preceeding 3 weeks, acute cardioversion can be considered as an alternative to rate control. On the contrary, in non-anticoagulated patients, rate control is generally the most appropriate strategy. In the acute setting, the target ventricular rate should usually be 80–100 bpm [4]. Once rhythm control is achieved and anticoagulation started, the patient can be considered for discharge and referred to a cardiologist or a general practitioner. If adequate rate control is not achieved, the patient is still symptomatic or if new ventricular dysfunction is to be detected, cardioversion should be considered: the patient must be fully anticoagulated independently of the thromboembolic risk (start with UFH or LMWH for immediate action and bridge then with vitamin K antagonists; novel anticoagulants may be used as alternative), then undergo a transoesophageal echocardiogram (TEE) to exclude intracardiac thrombi and finally electrically cardioverted. Anticoagulation should be continued at least 4 weeks or indefinitely, according on the thromboembolic risk profile. If thrombosis is detected, the patient should undergo another TEE after at least 3 weeks of effective anticoagulation and if it has resolved undergo ECV thereafter [4, 5, 10].
According to the Canadian Guidelines 2014 [10], the TEE-based CV approach should also be applied for a AF patients presenting <48 h after AF onset if high-risk features like valvular AF or stroke/TIA in the last 6 months are present.
5.
Antithrombotic therapy
It is generally believed that antithrombotic therapy is the mainstay of AF management in any care setting, as from this therapy, most of prognostic benefit in terms of morbidity (stroke prevention) and mortality has to be gained. Nevertheless, it is still much underprescribed, usually in less than 55 % of AF patients, as results from studies are conducted in different settings. EDs constitute excellent opportunities to start thromboprophylaxis in the absence of contraindications and help filling this gap [3]. Treatment options include LMWH or UFH, vitamin K antagonists (VKAs) and novel oral anticoagulants (NOACs).
Immediate anticoagulation can be obtained with unfractionated heparin (i.v. bolus of 80 UI/kg followed by continuous i.v. infusion adjusted to an activated partial thromboplastin time of twice the control value (initially 18 UI/kg/h)) [28] or LMWH (most popular being enoxaparin 100 UI/kg BID) based on ACUTE II [29] ad ACE trials [30]. Heparin therapy is generally used as bridging with VKAs which have a delayed onset of action which takes some days.
At present, three NOACs have been tested for AF cardioversion (prospectively or in post hoc analysis) [31–33]:
Dabigatran 150 mg twice daily (TD) if creatinine clearance > 50 ml/min, 110 mg TD if CrCl between 30 and 50. In the USA, the FDA approved 150 mg TD if CrCl >30 and 75 mg TD if ClCr 30–15. Do not use in association with dronedarone; reduce dose if concomitant verapamil is used.
Rivaroxaban 20 mg once daily or 15 mg if CrCl 49–30 ml/min.
Apixaban 5 mg TD or 2.5 mg TD if two or more risk factors for bleeding: age <80 years, weight <60 kg, creatinine >1.5 mg/dl.
Time to peak action is around 3 h for all three.
Valvular AF carries a very high risk per se and must always be treated with VKAs. NOACs were not tested or resulted inferior to VKAs in this setting [5, 34].
For patients with nonvalvular AF, a number of risk stratification schemes have been proposed, the mostly recommended [1, 5] being the CHA2DS2-VASc scoring system [35] (Table 5.1). As chronic therapy, anticoagulation is not indicated for score = 0, indicated for score ≥2, recommended for score = 1 by European guidelines [4], while US guidelines [5] indicate alternatively oral anticoagulants, aspirin or no antithrombotic treatment (Class IIb). We favour oral anticoagulation for patients with score = 1 unless at risk of bleeding.
Table 5.1
CHA2DS2-VASc scoring system
Acronym | Risk parameter | CHA2DS2VASc score | Stroke rate (%/year) |
|---|---|---|---|
C | Cardiac failure or dysfunction | 0 | 0 |
H | Hypertension | 1 | 1.3 |
A (2) | Age >75 | 2 | 2.2 |
D | Diabetes | 3 | 3.2 |
S (2) | Stroke/TIA or thromboembolism | 4 | 4 |
V | Vascular disease | 5 | 6.7 |
A | Age 65–74 | 6 | 9.8 |
Sc | Sex category (female) | 7 | 9.6 |
8 | 6.7 | ||
9 | 15.2 |
Any type of AF (paroxysmal, persistent, permanent) carries a similar risk of stroke, so anticoagulation should be chosen regardless of AF type. Atrial flutter has shown to have similar thromboembolic risk as AF and should be managed the same way.
All patients with AF >48 h duration (including those with CHA2DS2-VASc score = 0) candidate to cardioversion must be adequately anticoagulated for at least the three preceding weeks before the procedure, unless intraatrial thrombus has been ruled out by TEE or in case of rare emergency situations and continued on anticoagulation for at least 4 weeks afterwards. Lifelong anticoagulation is indicated for patients at high risk of stroke or AF recurrence.
For patients with AF onset <48 h, current guidelines [4, 5, 10] indicate cardioversion without previous anticoagulation, as former studies demonstrated low embolic complications (0.8 % stroke at 30 days in the study of Weigner et al. [36]). Some concern has raised as it was demonstrated that 4–14 % of these patients had an intraatrial thrombus on transoesophageal echocardiography [37, 38], and ESC 2010 guidelines [4] recommend to start anticoagulation with UFH or LMWH before cardioversion even for AF lasting <48 h. In high-risk patients, heparin therapy has to be followed by long-term oral anticoagulation (class I recommendation), while in patients with no risk factors, heparin therapy may be considered only pericardioversion (IIb, level C recommendation), but this latter recommendation is not evidence based. The 2014 AHA/ACC/HRS guidelines [5] make similar recommendations on use of peri- and postcardioversion antithrombotic therapy, adding the option to use NOACs for the purpose (Class I recommendation). In this context, also a recent European Expert Consensus [28] agrees with the use of NOACs with a class IIa recommendation. A recent large cohort study [39] on 7660 cardioversions of AF lasting <48 h supports the recommendation of prolonged anticoagulation in high-risk patients after CV: without anticoagulation, thromboembolic risk resulted 0.7 % at 30 days. Risk factors were age, heart failure, diabetes, and female gender (elements of CHA2DS2-VASc score system). Patients without risk factors experienced a very low complication rate of 0.2 %, whereas those with multiple risk factors had a thromboembolic risk approaching 10 %.
For patients with AF lasting >48 h or of unknown origin with no risk factors (CHA2DS2-VASc = 0), anticoagulation is not advised unless a delayed cardioversion is planned.
6.
Decision for cardiologist consultation or hospitalisation
Consultation with a cardiologist should be requested for:
Unstable patients (hypotension, angina, heart failure)
Patients with known significant cardiac disease
If significant underlying heat disease is suggested by objective findings, laboratory data or diagnostic testing
If rate or rhythm control has not been achieved
If cardiovascular complications have occurred as consequence of antiarrhythmic or rate control therapy
If cardioversion preceded by TEE is the choice
If Brugada pattern emerges on ECG after pharmacological cardioversion
Most of these patients will also require hospitalisation, as well as patients with concomitant acute illness (sepsis, pneumonia, pulmonary embolism, etc.).
5.3 What Cardiologists Should Know
“Pill-in-the-pocket approach”: useful treatment strategy for patients with no or minimal heart disease, no conduction disturbances and infrequent but prolonged and well tolerated AF recurrences. Patients have to take orally 200–300 mg flecainide or 450–600 mg propafenone (the higher dosage for weight >70 kg). This approach should be prescribed by a cardiologist after full diagnostic workup (and therefore in the author’s opinion avoided in first diagnosed AF) and must first be tested in the ED for safety and efficacy before it can be used at home. If this strategy is the choice, the cardiologist should specify this indication on his written evaluation of the patient so that instructions can be promptly be followed in the ED for the first test treatment. In the original article by Alboni et al., [40] rapid 1:1 flutter complicated only 1 case (0.6 %), so ESC 2010 guidelines [4] recommend this strategy as it was described, but AHA/AC/HRS 2014 [5] and CCS 2010 [41] guidelines recommend addition of beta blocker or nondihydropyridine calcium channel blocker to avoid this side effect. This strategy should not be used for patients with AFL.
Patients already on chronic antiarrhythmic therapy may present to the ED with relapsing acute onset AF. If cardioversion is judged to be the choice, we suggest electrical CV as first choice. Alternatively, pharmacological cardioversion (PCV) with the same antiarrhythmic agent administered intravenously at reduced dose can be performed although there is little evidence in literature. In our centre, it has been common practice for more than a decade to use flecainide or propafenone half dose bolus (1 mg/kg in 10 min) on top of chronic therapy with the same agents or intravenous amiodarone with conventional dosages and infusion rates on top of chronic amiodarone therapy, after checking for contraindications. We have not yet experienced significant side effects. Patients are discharged on the same antiarrhythmic agent, even at higher dose, as single or few AF relapses should not be interpreted as treatment failures if the antiarrhythmic burden is lowered on medication. We advise in general against the use of different antiarrhythmic drugs on top of chronic antiarrhythmic drug therapy although limited experience on intravenous ibutilide in patients taking amiodarone, propafenone or flecainide seems safe [42, 43].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







