24
Wound care
Introduction
Wound management forms a large percentage of the Emergency Department (ED) nurse’s workload, with some 8.5 % of the almost 12 million patients attending EDs in the UK in 2008/09 given a primary diagnosis of laceration (>600 000) and/or contusions or abrasions (>400 000) (NHS Information Centre for Health and Social Care 2011).
Anatomy of the skin
The skin is the largest external organ, and in adults weighs between 2.7 and 3.6 kg. It covers the whole of the body and its thickness varies around the body, with areas of greatest friction, such as the soles of the feet, being thickest and areas of low friction, like eyelids, being the thinnest (Tortora & Grabowski 2003). It also receives one-third of the body’s circulating blood volume – an oversupply compared to its metabolic needs (Baronski & Ayello 2008). The skin has five primary functions (Box 24.1):
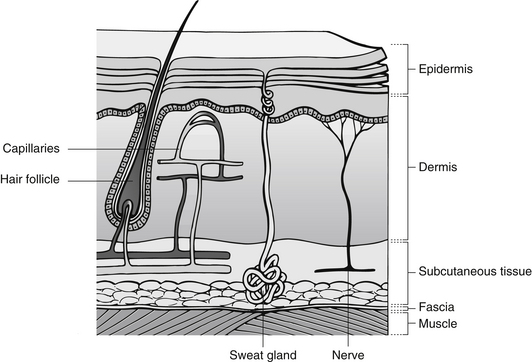
The skin is made up of two main parts, the epidermis and the dermis, which cover the subcutaneous fat layer and deep structures (Fig. 24.1) (Seidel et al. 2006).
Epidermis
This is subdivided into five distinct layers. Working from the surface, they are listed below.
Stratum corneum
This outer layer consists of keratinized cells that assist with resisting thermal, chemical and mechanical stress and restricts water loss, making the skin susceptible to maceration if constantly exposed to water. These cells shed continuously at a rate of 1 500 000 per hour. The primary function of this layer is to act as a barrier (Seidel et al. 2006).
Stratum lucidum
This layer of dead cells is found in areas needing extra protection, such as the soles of the feet and the palms of the hands (Carville 2007).
Stratum granulosum
This middle layer contains granular cells containing cytoplasmic granules which are the precursor to keratin. As they migrate from the lower level to this level the cells flatten out and the nuclei die (Carville 2007).
Stratum spinosum
These form a layer of living cells that act as intracellular bridges preventing cell separation. Several different cell types exist in this layer including Langerhans’ cells which are dendritic cells involved with antigen processes (Murphy et al. 2005).
Stratum basale (or stratum germinativum)
This layer lies next to the dermis. These cells are responsible for germination of new epithelial cells and are reliant on the dermis for nutrients from the blood supply. Melanocytes are found in this layer and produce melanin which is mainly responsible for skin tones (Murphy et al. 2005).
Dermis
The dermis contains blood vessels, nerves, sebaceous glands and hair follicles and is made up primarily of collagen and elastin. This gives strength and elasticity to the skin (Copstead & Banasik 2005). Sensory nerves located in the dermis provide sensations of touch, temperature and pain. Cells in the dermis include:
• fibroblasts – used in wound healing; they lie between bundles of collagen and act to synthesize elastin and collagen
• tissue macrophages – these phagocytic cells engulf debris and matter during healing
• tissue mast cells – found near hair follicles and blood vessels, these cells produce histamine and heparin.
Between the epidermis and dermis is the basement layer that is an acellular, non-vascular and non-innervated membrane separating the two layers of skin. This membrane provides support to the skin and plays a role in the movement of nutrients between layers (Carville 2007).
The hypodermis is found under the dermis and is composed of adipose tissue, connective tissue and blood vessels. It provides insulation, shock absorption and is responsible for temperature regulation and storage of lipids (Seidel et al. 2006).
Wound healing
Terminology and the number of stages in the healing process vary between texts, however, the general consensus is that four phases of healing occur. They usually follow a set pattern (Clark 2002), but can occur concurrently, and different parts of the same wound can heal at different rates (Table 24.1). Wound healing can be complex and is affected by the mechanism of injury and the general health of the patient (Gantweker & Hom 2012).
Table 24.1
| Duration | Phase | Signs |
| First hour | Haemostasis | Initial vasoconstriction Coagulation of wound |
| 10 minutes–5 days | Inflammatory | Vasodilation, pain, heat, swelling |
| 3 days–1 month | Proliferation | Wound size diminishing |
| Surrounding skin of normal colour | ||
| Less pain | ||
| 3 weeks–1 year | Maturation | Wound healed Scarring fades |
Haemostasis
The body’s initial response to a cut in the skin is bleeding. This extravasation initiates platelet activity and coagulation of blood. It also results in vasoconstriction and release of histamines and ATP, which also attract leucocytes. Platelets begin to aggregate and the coagulation cascade results in the development of a fibrin mesh and clot, or beginnings of a scab, which temporarily seals the wound. Once the clot is formed, fibrinolysis commences as part of the body’s defence mechanism. This ensures the clot does not extend and allows better migration of cells into the wound bed (Baranoski & Ayello 2008).
Inflammatory stage
This is to enable plasma to leak into tissues around the area of injury. This creates wound exudate. Neutrophils are the first leucocytes that usually arrive within 6–12 hours at the injury site (Lewis et al. 2011a), leak into the area of the wound and offer initial protection from infection by engulfing and digesting bacteria. Neutrophils have a short life span, and so are replaced by monocytes that are capable of phagocytosis. These promote new tissue formation and angiogenesis, and continue to engulf and destroy bacteria and debris from the wound, including old neutrophils (Baranoski & Ayello, 2008).
• redness – because of local vasodilatation
• heat – because of increased blood supply and metabolic activity
• oedema – because increased capillary permeability allows fluid to leak into the extracellular space
• pain – due to pressure of fluid in tissues and chemical irritation from enzymes such as prostaglandin.
This inflammation is vital to the natural healing. If it is suppressed by drugs or illness, healing will be delayed. Macrophages are essential for transition into the proliferation stage of healing, as they begin to produce transforming growth factor (TGF), which promotes angiogenesis and the formation of new tissues. Macrophages also produce fibroblast growth factor (FGF), which stimulates fibroblast production (Bale & Jones 2006).
Proliferation stage
This starts 3–5 days post-injury and can last up to three weeks (Lewis et al. 2011b). As its name suggests, this part of the healing process is about growth and reproduction of tissue to replace that lost in injury. By day five, the wound surface will only be 7 % of its pre-injury tensile strength (Waller & Tan 2009). In order to produce new tissue, the wound needs a good oxygen supply and essential nutrients such as vitamin C, protein and zinc (Kumar et al. 2005, Bishop 2008). As angiogenesis occurs in response to wound hypoxia and TGF, new capillary loops develop and the wound is oxygenated. Three distinct processes occur during the proliferation phase.
Granulation
This is the formation of new tissue up from the base and in from the sides of a wound. It is dependent on the division of endothelial cells forming new capillary loops, until eventually they meet up with existing undamaged blood vessels. At the same time, fibroblasts begin to produce a network of collagen and ground substance that fills tissue spaces and begins to bind fibres together. Collagen synthesis depends on adequate nutrients, i.e., vitamin C, copper and iron (Kumar et al. 2005). These can usually be obtained from a healthy diet. Collagen forms in a haphazard and jelly-like structure, and with adequate vitamin C matures into a strong cross-linked structure which gives the tissue its tensile strength.
Contraction
This occurs at the same time as epithelialization. In wounds where tissue loss has occurred, once the wound bed has filled with healthy granulation tissue, myofibroblasts develop which contract and pull the wound edges together, therefore decreasing the overall size of the wound. Keratinocytes are responsible for the re-epithelialization from the wound edges (Naude 2010).
Epithelialization
As the wound cavity is filled with granulation tissue and the surface is regenerated with epithelial cells, the proliferation stops. If this does not happen, e.g., if overgranulation occurs due to continued hypoxic stimulation, perhaps as a result of local ischaemia, then excessive scar tissue is formed (Kumar et al. 2005).
Maturation or remodeling stage
This begins around three weeks after injury, and is a process of returning the area to its usual functional structure. The process is twofold.
Firstly, collagen is remodelled, sometimes over a period of years. The aim of this is to gradually replace newly formed type III collagen, laid down in the proliferation phase, with stronger, more organized collagen fibres. The amount of collagen does not change; its bundles become thicker and shorter and hold the wound together more tightly. Although the skin and wound scar become stronger, the area only usually regains about 80 % of the pre-injury tensile strength (Kumar et al. 2005). This takes a long time; at thee months post-injury 50 % of tensile strength is considered good healing (Baranoski & Ayello 2008).
The second part of the process is the rationalization of blood vessels bringing extra nutrients to the area. This process occurs gradually, and its progression can be monitored by the gradual fading of the scar. It will become paler and flatter as blood vessels diminish. Once maturation is achieved the scar will appear white; it is avascular, has no sebaceous glands and no hairs (Baranoski & Ayello 2008).
Scarring
Dermal damage results in an abnormal formation of connective tissue. This is permanent and manifests as a scar on the skin surface. Scarring follows three phases, although the time span increases with age, skin pigmentation and as a result of poor general health (Table 24.2). Certain areas of the body are notorious for poor scarring – the shoulder, knee, and sternal areas, which are areas under a lot of tension and motion (Bayat et al. 2003, Capellan & Hollander 2003).
Table 24.2
| No. of weeks post-injury | Scar characteristics |
| 0–4 weeks | Soft, weak scar line |
| 4–12 weeks | Scar contracts, becomes harder and stronger |
| 12–52 weeks | Scar line flattens and becomes soft and supple, moving easily with surrounding skin. Gradually whitens as vascularity decreases. Skin does not regain pre-injury elasticity |
Keloid scarring is usually a genetic phenomenon where collagen type I is produced in a tumor-like fashion with uncontrolled growth of scar tissue (Widgerow 2011). Keloid scarring results from the formation of large amounts of scar tissue in the proliferation stage of healing. It results from an increase in collagen synthesis and lysis to an extent where tissue formation exceeds cell breakdown (Bryant & Nix 2006). Keloid scarring is also considered to be related to the melanocyte-stimulating hormone as it is much more common in people with heavily pigmented skin, predominantly those aged 10–30 years (Bayat et al. 2003, Mustoe 2004). Tissue growth is persistent, with scarring often being much larger than the original wound. Early effective wound management can reduce the risk of keloid scarring.
Factors affecting wound healing
Although patients with sudden traumatic wounds do not have the same physiological and educational preparation as patients undergoing surgery, many of the influences on wound healing can be optimized by effective education and empowerment during their initial visit for wound management. Clinical factors affecting healing potential can also be identified at this early stage, and the patient’s care can be designed to accommodate them. The main influences on wound healing are listed in Box 24.2.
Nutrition
• poor healing with reduced tensile strength and an increased risk of wound dehiscence (Scholl & Langkamp-Henken 2001)
• an increased likelihood of infection (Lansdown 2004)
Protein and calorie intake need to be above normal recommended levels to support additional collagen synthesis and metabolic activity (Table 24.3).
Table 24.3
Calorie and protein intake in wound healing
| Energy (kcal) | Protein (g) | |
| Men | 2150–2510 | 54–63 |
| Women | 1680–2150 | 42–45 |
Vitamin deficiency
Vitamin C is essential for the synthesis of collagen; a deficiency reduces wound tensile strength, increases the fragility of capillaries and impairs angiogenesis. Vitamin A supplement improves healing in patients on corticosteroids (Scholl & Langkamp-Henken 2001). It can help to restore inflammatory response and reduces the risk of wound infection. Similarly, a vitamin A deficiency increases infection risk. Vitamin B complex is necessary for wound strength as it contributes to cross-linking of collagen fibres. Vitamin K is essential for the clotting process in early wound healing (Lansdown 2004).
Trace element deficiency
Iron deficiency has two significant impacts on wound healing: first, in patients with anaemia, oxygen transportation is reduced and therefore tissue perfusion is inhibited; and second, iron is a necessary co-factor in collagen synthesis. Copper deficiency is rare but where it occurs, enzyme activity is restricted and collagen cross-linkage is impaired. Zinc deficiency delays wound healing because it slows collagen synthesis, reduces wound strength and decreases speed of epithelialization (Baranoski & Ayello, 2008). The use of a dietician may be of benefit to patients with nutritional issues.
Body type
Body type may also affect wound healing. An obese patient, for example, may experience a compromise in wound healing due to poor blood supply to adipose tissue. In addition, some obese patients have protein malnutrition, which further impedes the healing. Conversely, when a patient is emaciated, the lack of oxygen and nutritional stores may interfere with wound healing (Thomas Hess 2011).
Wound assessment
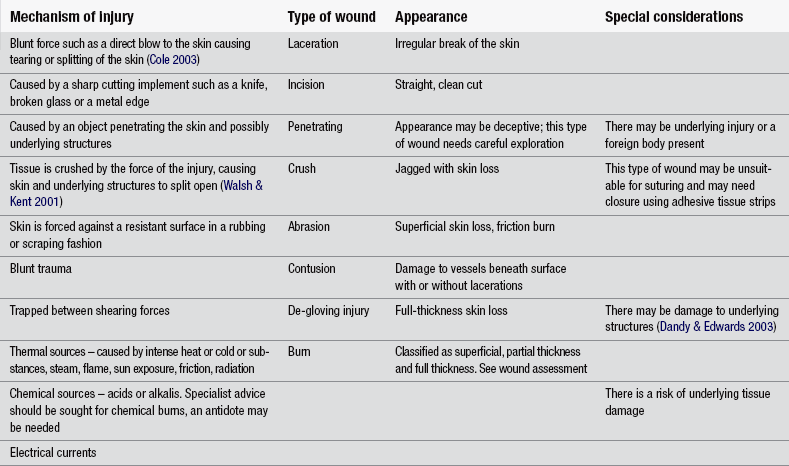
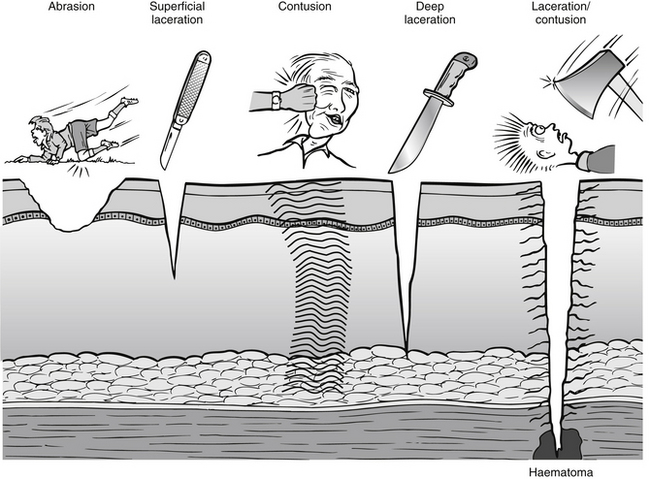
It is essential that an accurate history is elicited from the patient to ensure systematic assessment and appropriate management of the wound. As with all patients attending the ED, the immediate history of events leading up to ED attendance is imperative. Assessment should consider when, where and how the injury occurred. The mechanism of injury gives important clues to the type of wound being dealt with as well as any materials involved in the injury, such as wood splinters, glass, metal, etc. (Flarity & Hoyt 2010). Table 24.4 relates the mechanism of injury to wound type. The type of skin damage of injury is usually related to the mechanism of injury (Fig. 24.2).
Table 24.5 demonstrates the essential principles of wound assessment.
Table 24.5
| Assessment question | Rationale |
| How did the wound occur? | To establish mechanism of injury and classification of wound To exclude serious or other injuries To assess potential contamination risk (Clark 2004) |
| When did the injury occur? | To ensure it is appropriate for primary closure To assess for infection risk (wounds >6 hours old are more prone to infection) |
| Size, site and depth of wound | To ascertain the most appropriate method of wound closure (e.g., wounds over joints or requiring high tensile strength usually require sutures) (Autio & Olson 2002) To ensure base of wound can be visualized |
| Past medical history | To detect pathology that may delay or influence healing |
| Medication history | To detect medication that may delay or influence healing To avoid prescribing contraindicated medications as part of wound management |
| Allergies | To avoid allergic reactions during the wound management process and during subsequent treatment |
| Tetanus status | To ensure the patient has tetanus immunity |
| Occupation and dominant hand | To assess the effect of the injury on the patient’s lifestyle To ensure treatment is appropriate for the patient’s lifestyle |
Wound examination
Effective wound examination should reveal the extent of tissue damage, the degree of contamination and specifically the integrity of the nerves, tendons and vascular supply (Autio & Olson 2002, Clark 2004). It should also exclude the presence of foreign bodies (FBs). All findings should be documented, including the normal ones.
Excessive bleeding and macerated or badly damaged tissue can detract from a thorough examination. Bleeding should be controlled to allow an accurate examination to be carried out (Clark 2004). Assessment of vascular integrity should include the patient’s estimation of blood loss, together with objective evidence of haemorrhage. The wound should be carefully inspected for continuous oozing of blood (suggestive of venous bleeding), spurting of bright-red blood (indicative of arterial injury) and haematoma formation, which could pose a risk to healing in the form of potential infection. Haemostasis is usually achieved through direct pressure and elevation of the injured area. Where bleeding cannot be controlled specialist input should be sought. Vascular integrity distal to the wound can be assessed by observing skin colour distally to the wound, feeling skin temperature, and checking distal pulses and the speed of capillary refill (McKenna 2006).
Nerves have both a sensory and a motor function and therefore both can be checked to eliminate injury. This assessment should occur before local anaesthetic is used. Sensory function distal to the wound should be assessed either by use of a cotton wool wisp to detect the absence or presence of sensation, or by gentle pinprick tests to assess sharp and dull sensation. Motor function should be assessed, particularly in hand or wrist injuries, and this can be done by assessing a variety of movements of the patient’s hand and wrist (McKenna 2006).
Tendon injury should also be identified and eliminated as part of the examination stage of wound management. Tendons can often be partially severed and still retain their function, so elimination of this type of injury should be done in two ways. An initial systematic examination of the patient’s function in the affected limb may demonstrate reduced power or function. This should be followed by direct visualization of the wound to discover any structural damage (Waller & Tan 2009
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree