Ventricular Diastolic Dysfunction
Robert A. Taylor
Carlos A. Roldan
Ventricular diastole includes four phases: (i) left ventricle (LV) relaxation or pressure fall (from aortic valve closure to mitral valve opening), (ii) rapid filling (after mitral valve opening), (iii) diastasis (passive low filling), and (iv) atrial contraction.
Ventricular diastole depends on elastic recoil, ventricular energy-dependent relaxation, compliance, and atrial pressure.
Ventricular filling is influenced by volume status, diastolic filling time, wall tension, and contractility and therefore is altered by preload, heart rate, afterload, and systolic function.
Increased chamber stiffness or diastolic dysfunction prevents adequate filling of the ventricles at normal atrial pressures and is due to impaired relaxation, decreased compliance, or commonly both. These abnormalities occur over a continuum.
Elevation of LV end-diastolic pressure (LVEDP) and right ventricular (RV) end-diastolic pressure (RVEDP) of 16 mm Hg and 12 mm Hg, respectively, is seen in diastolic dysfunction independent of systolic function.
Diastolic dysfunction in the absence of systolic dysfunction is the cause of clinical heart failure (CHF) in 30% to 40% of patients (1).
Mortality curves for systolic and diastolic CHF are virtually identical, both demonstrating a poor prognosis regardless of etiology (2).
Asymptomatic diastolic dysfunction is common by Doppler echocardiography (echo) and is also a poor prognostic indicator (3,4).
The history and physical examination have low sensitivity for the detection of diastolic dysfunction and is limited in distinguishing systolic from diastolic CHF.
Common Etiologies
Clinical or subclinical LV diastolic dysfunction is more prevalent as an isolated abnormality than mixed systolic and diastolic dysfunction. However, most, if not all, patients with systolic dysfunction also have diastolic dysfunction.
Controlled Doppler echo series in asymptomatic patients with risk factors for diastolic dysfunction have shown that LV diastolic dysfunction (grade 1 and uncommonly grade 2) is at least as prevalent as clinical diastolic dysfunction (4,5).
Isolated LV diastolic dysfunction: The most common causes are aging, hypertension with and without LV hypertrophy, and coronary artery disease (CAD) (Table 4.1).
Mixed LV systolic and diastolic dysfunction (Table 4.1): All disease processes causing LV systolic dysfunction cause variable degrees of diastolic dysfunction due to the effects of myocardial disease or ventricular dilatation on LV compliance. Hypertension, microvascular or epicardial CAD, and valvular heart disease are the predominant causes.
The most common causes of diastolic dysfunction of the RV are pulmonary hypertension of any etiology (for which left heart disease is probably the most common cause) and RV ischemia or infarction (6) (Table 4.2). However, several conditions that affect the LV also affect the RV (Table 4.1). Isolated infiltrative RV diseases are uncommon.
Table 4.1 Causes of left ventricular diastolic dysfunction | ||||
|---|---|---|---|---|
|
Echocardiography
Indications for Echocardiography in Patients with Diastolic Dysfunction
General indications for Doppler echo in the evaluation of patients with suspected or known ventricular diastolic dysfunction include the following:
Evaluation of symptomatic patients to detect and assess the severity of ventricular diastolic and systolic dysfunction.
To determine the cardiac etiology of ventricular diastolic dysfunction.
To assess the prognosis of patients with ventricular diastolic dysfunction.
To assess the short- and long-term response to therapy for diastolic dysfunction.
Table 4.2 Causes of right ventricular diastolic dysfunction
Diseases causing left atrial or pulmonary venous hypertension
Left heart diseases leading to elevated LVEDP and pulmonary hypertension (see Table 4.1)
Congenital pulmonary vein stenosis
Pulmonary veno-occlusive disease
Diseases of the pulmonary parenchyma
Chronic obstructive lung disease
Restrictive lung diseases
Infiltrative/granulomatous diseases
Cystic fibrosis
Upper airway obstruction
Diseases of the pulmonary arteries
Primary pulmonary hypertension
Toxin induced (i.e., anorexic agents)
Vasculitides
Congenital heart disease (atrial or ventricular septal defects and patent ductus arteriosus)
Infection
HIV
Diseases of the thoracic cage and neuromuscular system
Obesity-hypoventilation/sleep apnea
Pharyngeal-tracheal obstruction
Kyphoscoliosis
Pleural fibrosis
Neuromuscular disorders
Nonvasculitic diseases resulting in pulmonary artery obstruction
Acute and chronic thromboembolism
Hemoglobinopathies (e.g., sickle cell disease)
Primary or metastatic malignancies
Peripheral pulmonic stenosis
Congenital pulmonary hypoplasia
LVEDP, left ventricular end-diastolic pressure.
M-Mode and Two-Dimensional Echocardiography: Assessment of the Morphology of the Left and Right Heart Chambers
Best Imaging Planes
Transthoracic echocardiography (TTE): Parasternal long-and short-axis and apical views.
Table 4.3 Appropriate (score 7–9) indications for Doppler echocardiography in patients with suspected or known ventricular diastolic dysfunction
Symptoms of dyspnea or shortness of breath.
Abnormal chest radiography, electrocardiogram, or elevation of serum BNP.
Suspected hypertensive heart disease.
Known or suspected systolic or diastolic heart failure.
Re-evaluation of known systolic or diastolic heart failure to assess response to or guide therapy.
Evaluation of left ventricular systolic and diastolic function following acute MI.
Re-evaluation of left ventricular systolic and diastolic function following MI during the recovery phase to assess response to or guide therapy.
Evaluation of known or suspected hypertrophic cardiomyopathy.
Re-evaluation of known hypertrophic cardiomyopathy in a patient with a change in clinical status to assess response to or guide therapy.
Evaluation of a suspected restrictive, infiltrative, or genetic cardiomyopathy.
Baseline and serial regular re-evaluations in patients undergoing cardiotoxic therapy.
Evaluation of known or suspected pulmonary hypertension for estimation of pulmonary artery systolic and diastolic pressures and evaluation of right ventricular systolic and diastolic function.
Evaluation of respiratory failure with suspected cardiac etiology.
Evaluation of patients with suspected acute pulmonary embolism to guide the decision of thrombectomy or thrombolytic therapy.
BNP, B-type natriuretic factor; MI, myocardial infarction. (Adapted from Douglas PS, Garcia MJ, Haines DE, et al.
ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. J Am Coll Cardiol. 2011;57:1126–1166.)
Transesophageal echocardiography (TEE): Transgastric long- and short-axis and midesophageal four and two chamber views.
Diagnostic Methods and Formulas
Measurements of LV dimensions are made just below the mitral valve leaflet tips (between the mitral valve and papillary muscles).
Measurements of the interventricular septum (IVS) and posterior wall (PW) thicknesses are made below the mitral valve tips at end diastole.
Measurements of IVS, PW, and LV end-diastolic dimension (LVEDD) are used to calculate LV mass by the following formula (8): 0.80 × 1.05 × (IVS + PW + LVEDD)3 – (LVEDD)3.
Since body size and gender affect LV thickness and mass, calculation of LV mass indexed to body surface area (BSA) using the area-length (AL) or truncated ellipsoid (TE) methods is recommended (8,9):
LV mass (A-L) = 1.05 {[5/6 A (a + d + t)] – [5/6 A (a +d)}
LV mass (TE) = 1.05 × {(b + t) [2/3 (a +1) + d – d/3(a + t)] – b [2/3 a + d – d/3a]}, where A1 indicates the total LV area; A2, LV cavity area; a, long-axis dimension; b, short-axis radius; d, truncated semimajor axis; and t, mean wall thickness.
Normal LV mass values indexed to BSA are 44 to 88 g/m2 for women and 50 to 102 g/m2 for men.
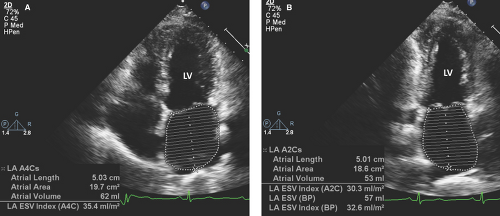
Measurements of the anteroposterior dimension of the left atrium (LA) are made from the parasternal long-axis view at end systole using M-mode or two-dimensional (2D) images.
Two-dimensional imaging from the apical four- and two-chamber views with planimetry allows measurement of the LA volume using the A-L technique:
Two-dimensional imaging is essential for proper sample placement of pulsed and continuous wave, tissue, and color Doppler imaging.
Key Diagnostic Features (General)
An LA dimension >4.5 cm provides evidence of LV diastolic dysfunction independent of LV ejection fraction (LVEF).
LA dimensions show a direct relationship with calculated measurements of LA volumes indexed to patient BSA.
Key Diagnostic Features for Specific Conditions
CAD and ischemic cardiomyopathy:
Wall motion abnormalities associated with thinning or scarring are highly specific for ischemic heart disease as a primary or contributing factor to LV diastolic dysfunction independently of LVEF.
Acute or chronic myocardial ischemia results in diastolic dysfunction ranging from impaired LV relaxation to restrictive filling.
Symptoms of CHF in ischemic cardiomyopathies correlate highly with echo measures of elevated LVEDP (13).
Hypertensive heart disease:
Echo evidence of LV hypertrophy, increased LV mass, and diastolic dysfunction occurs well in advance of systolic dysfunction.
Increase in LV wall thickness (>1.1 cm in men and 1.0 cm in women) or LV mass indicate LV hypertrophy due to systemic hypertension, hypertrophic cardiomyopathy, or infiltrative diseases.
Because of the high prevalence of hypertension, especially in the older population, hypertensive heart disease is the most common abnormality leading to diastolic heart failure.
Restrictive cardiomyopathy:
Normal ventricular dimensions, biatrial enlargement, and usually preserved systolic function.
Findings are almost exclusively of severe diastolic dysfunction.
A restrictive filling pattern and LA enlargement in a patient with a normal LVEF are associated with a poor prognosis similar to that of a restrictive pattern in dilated cardiomyopathy.
Infiltrative heart diseases:
Amyloidosis:
Normal systolic function without LV or RV dilatation.
Pronounced speckling and hypertrophy of the myocardium (often referred as “ground glass” appearance) is seen. The valvular structures may demonstrate a similar pattern.
Pericardial effusions may also be present.
Sarcoidosis:
Infiltration of the myocardium with noncaseating granulomas produces LV dilatation and regional wall motion abnormalities (especially of the mid- and basal segments), commonly misinterpreted as CAD.
Endomyocardial fibrosis:
Fibrotic and thrombotic (echodense) thickening of the endocardium, particularly at the LV and/or RV apices.
The degree of diastolic dysfunction is usually severe with restrictive features.
Hypertrophic cardiomyopathy:
Asymmetric severe hypertrophy with normal to hyperdynamic LV systolic function and dynamic subaortic outflow obstruction manifested by systolic anterior motion of the mitral valve and mitral regurgitation are characteristic.
Pulmonary hypertension and cor pulmonale:
Chronic moderate or worse (>45 mm Hg) pulmo-nary hypertension leads to cor pulmonale, defined as RV hypertrophy and dilatation, diastolic dysfunction, and, over time, systolic dysfunction (6,14).
Abnormal septal motion of RV pressure overload (toward the LV) during late systole and early diastole is characteristic.
Quantitatively, RV dilatation is defined as a basal end-diastolic diameter >42 mm, midcavity end-diastolic
diameter >35 mm, and/or a longitudinal (from the tricuspid annulus level to the RV apex) end-diastolic diameter >86 mm (6,14). However, these cutoff values may be highly specific but not sensitive.
An RV size greater than or equal to the LV suggests severe RV dilatation independently of RV dimensions.
The downward systolic excursion of the lateral tricuspid annulus assessed by M-mode from the four-chamber view <1.6 cm indicates RV systolic dysfunction (15).
Dilation of the right atrium (RA) is present if the maximal minor dimension (parallel to the tricuspid annulus), major dimension (from the annulus to the superior RA wall), and RA area exceed 44 mm, 53 mm, and 18 cm2, respectively (6,14).
RA hypertension (≥10 mm Hg) is present when there is dilatation (≥1.5) of the inferior vena cava (IVC) and the IVC shows ≤50% collapse with inspiration (16).
Valvular heart disease:
Stenotic or regurgitant lesions must be at least of moderate degree to cause elevation of intracardiac pressures (17).
Left-sided valvular diseases commonly cause RV diastolic dysfunction by causing pulmonary hypertension.
Constrictive pericarditis:
The thickened and calcified pericardial sac causes a physical constraint to ventricular filling.
Septal motion is paradoxical, and the IVC is plethoric.
Respiratory variation of ≥25% and ≥40% in the transmitral and transtricuspid E velocities, respectively, are supportive findings of constriction.
Age-related changes:
Healthy elderly patients often display greater than or equal to mild atrial dilatation on 2D and M-mode images in association with impaired LV relaxation.
Cardiac transplant rejection:
The increase in myocardial stiffness results from organ rejection causing myocyte necrosis.
Abnormal LV and RV filling can be identified at an early stage in acute rejection using Doppler echo.
Pitfalls
Two-dimensional echo images do not allow for a functional assessment of the LV or RV filling properties.
M-mode echo does not allow characterization of myocardial diseases or functional assessment of ventricular filling.
Proposed cutoff values for enlargement of right heart chambers may be specific but not sensitive.
Doppler Echocardiography: Assessment of the Severity of Diastolic Function
Pulsed Wave Doppler
Mitral and Tricuspid Valve Inflow Patterns
Best Imaging Planes
TTE apical and TEE midesophageal four-chamber views with a sample volume of 1 to 3 mm placed between the mitral or tricuspid leaflet tips.
Doppler recordings should be obtained at end expiration, at a sweep speed of 50 to 100 mm/second, and averaged over three consecutive cardiac cycles.
TTE apical or TEE five-chamber views with the sample volume between leaflet tips and LV outflow tract (LVOT) for imaging of mitral valve inflow and aortic valve closure clicks to measure isovolumic relaxation time (IVRT) (period from aortic valve closure to mitral valve opening).
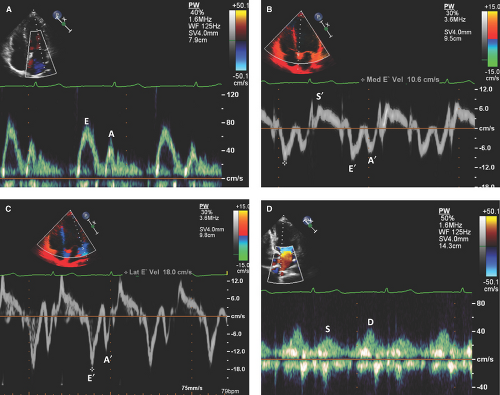
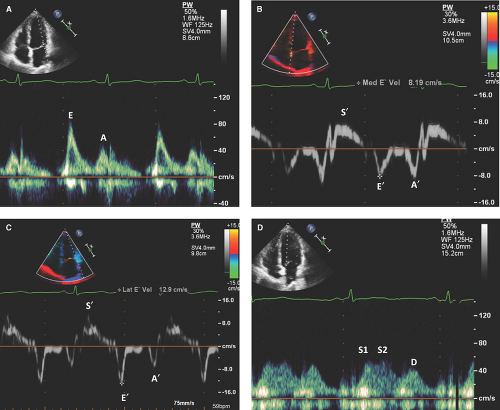
Doppler measurements of the mitral and tricuspid inflow velocities include (i) peak early filling velocity (E), (ii) late diastolic filling velocity (A), (iii) E/A ratio, (iv) E deceleration time, and (v) IVRT. (See Figs. 4.2A and 4.3A for normal mitral inflow patterns.)
Duration of A wave and rarely seen diastasis peak velocities are uncommonly measured.
Key Diagnostic Features
Left Ventricular Diastolic Dysfunction
The mitral or tricuspid valve E wave primarily reflects the LA-LV or RA-RV pressure gradient during early diastole and is affected by preload and alterations in LV or RV relaxation, respectively.
The mitral or tricuspid valve A wave reflects the LA-LV or RA-RV pressure gradient during late diastole and is affected by LV or RV compliance and LA or RA contractile function.
Based on the mitral inflow Doppler patterns, LV diastolic dysfunction is classified as (i) impaired relaxation, (ii) pseudonormalization, and (iii) restrictive filling (4,6,18).
Impaired relaxation (grade I or mild diastolic dysfunction):
The slow-falling ventricular pressure during isovolumic relaxation results in a prolonged time before it drops below the LA or RA pressure, a delay in the mitral or tricuspid valve opening, and consequently in a prolongation of the IVRT.
Prolonged IVRT, prolonged deceleration time (>220 msec) of E velocity, prolonged IVRT (≥110 msec), reduced E peak velocity (<50 cm/second), dominant
A velocity, and decreased E/A ratio (<0.8) are characteristic (4) (Fig. 4.4A).
Pseudonormalization (grade II or moderate diastolic dysfunction):
Decreased ventricular compliance results in increa-sed ventricular end-diastolic pressure, which then causes an increase in atrial pressure to preserve transvalvular (mitral or tricuspid) gradient.
Increased E velocity, usually but not always decreased A velocity, pseudonormalized E/A ratio (0.8 to 1.5), shortened deceleration time (160 to 220 msec), and shortened IVRT (<90 msec) are characteristic findings (Figs. 4.6 and 4.7).
Restrictive filling pattern (grade III or severe diastolic dysfunction):
Indicates significantly elevated LVEDP and LA pressure.