Key Clinical Questions
What is the clinical and prognostic significance of frequent premature ventricular complexes (PVCs) and nonsustained ventricular tachycardia (NSVT), and how should they be managed?
In the setting of a wide complex tachycardia (WCT), what features favor a diagnosis of ventricular tachycardia (VT)?
What types of VT or ventricular fibrillation (VF) can occur in the absence of structural heart disease, and what types can be precipitated by exercise?
How should a clinician distinguish between stable VT, unstable VT, and pulseless VT diagnostically and therapeutically?
Which patients deserve implantable cardiodefibrillators (ICDs) for primary or secondary prevention of sudden cardiac death (SCD), and what is the role of antiarrhythmic drug therapy in primary and secondary prevention of SCD?
Introduction
Ventricular arrhythmias are a heterogeneous group of cardiac rhythm disturbances that range from benign, asymptomatic premature ventricular complexes (PVCs) to malignant and often fatal arrhythmias, such as ventricular fibrillation (VF). They can occur in both the absence and the presence of overt heart disease and have a wide spectrum of clinical significance and prognostic implications. The risk of sudden cardiac death (SCD) with some of these arrhythmias has led the medical community to identify patients susceptible to such events, and thus justify potentially dangerous and expensive treatments. Fortunately, evidence gathered from clinical trials in the last 3 decades has helped establish clear epidemiological patterns for most types of ventricular rhythm abnormalities. These patterns have proven to be vital in the development of widely accepted, evidence-based guidelines for therapy and prevention.
This chapter describes the epidemiology, pathophysiology, clinical presentation, diagnosis, treatment, and prevention of the most commonly encountered ventricular arrhythmias in current hospital practice. Care has been taken to include and cite evidence when appropriate. References to guidelines from the American College of Cardiology (ACC), American Heart Association (AHA), European Society of Cardiology (ESC), and Heart Rhythm Society (HRS) are also found throughout the text.
Premature Ventricular Complexes
PVCs, also known as ventricular premature beats (VPBs), ventricular extrasystoles (VES), or ventricular premature depolarizations (VPDs), are a frequent form of ventricular arrhythmia that occurs in both the presence and the absence of heart disease. Their prevalence depends on various demographic factors such as age, the presence or absence of heart disease, and the method used to detect them. In the Atherosclerosis Risk in the Community (ARIC) study, a review of over 15,000 healthy patients, the overall prevalence of any PVC on a 2-minute electrocardiogram (ECG) was 6% with a 34% rise for every 5-year increment in age. Conversely, published studies of healthy individuals evaluated with 24-hour ambulatory monitoring have found the prevalence of PVCs to be as high as 80%. In the background of acute myocardial infarction (AMI), the prevalence of PVCs may rise up to 93%.
Despite theoretical concerns of PVCs as a risk factor for heart disease, the occurrence of PVCs in healthy subjects has not been shown conclusively to have an impact on morbidity or mortality. A subanalysis of the Multiple Risk Factor Intervention Trial (MRFIT) found a significant threefold increase in SCD among healthy white males with any PVC on a 2-minute rhythm strip. An analysis of the Framingham Heart Study data also found significant increases in mortality when frequent or complex PVCs were identified in 1-hour ambulatory monitoring or when PVCs were identified during exercise testing. Nevertheless, several other studies do not support an association between PVCs and cardiovascular events or mortality, and no consensus exists regarding the relevance of finding them in an otherwise healthy population. Interestingly, with the exception of frequent (>10/hour) and complex (primarily nonsustained VT) in post-myocardial infarction (MI) patients, PVCs alone do not seem to be an independent predictor of outcomes in patients with known heart disease such as left ventricular hypertrophy (LVH) and dilated or hypertrophic cardiomyopathy.
|
Symptoms related to PVCs are infrequent but important to recognize. The association of specific symptoms with PVCs, along with the perceived intensity of the symptoms, has important implications for treatment. Patients may complain of palpitations, which are usually due to postextrasystolic hypercontractility, or the feeling of a “missed beat” secondary to the postextrasystolic pause. Patients may also describe neck pulsations or lightheadedness. Rarely, frequent PVCs can decrease cardiac output and cause exacerbation of underlying heart disease, particularly coronary disease and congestive heart failure (CHF). Physical exam findings vary according to the hemodynamic and electrophysiological consequences of the premature beat. Jugular venous pulsation may reveal Cannon A waves (larger than normal, sporadic, jugular venous awaves) if there is atrial activation (normal sinus beat) simultaneous with ventricular activation by the PVC. If there is functional AV block, a variable intensity first heart sound may be heard. A PVC that has a right bundle branch block morphology (ie, originating in the left ventricle [LV]) will generate a widely split P2. Finally, murmurs vary predictably in relation with PVCs, and careful auscultation following the extrasystolic beat may provide important clues (decreased intensity in mitral valve prolapse murmur, increased dynamic obstruction in hypertrophic cardiomyopathy).
The mechanisms responsible for the generation of PVCs are similar to those causing most tachyarrhythmias. Although increased automaticity, reentry, and triggered activity can all be causes of PVCs, each mechanism may have some distinguishing features on the surface ECG. PVCs often occur with a predictable and constant relationship to the preceding sinus beat (ie, fixed coupling). When this is the case, the mechanism of PVC generation is usually reentry. Examples of this type of PVC include bigeminy (a PVC after every sinus beat), trigeminy (a PVC after every two sinus beats), and quadrigeminy (a PVC after every three sinus beats). When fixed coupling is not present, the mechanism responsible for the generation of the PVC is generally increased automaticity or triggered activity from one or more ectopic ventricular foci. A specific example of this type of PVC, parasystole, refers to a protected automatic ventricular focus that is not reset or altered by surrounding ventricular activation (ie, entrance block). Because the focus is independent of sinus activity and ventricular activation, there will be no fixed coupling but the interectopic intervals (interval between successive PVCs) will have a common denominator (Figure 128-1).
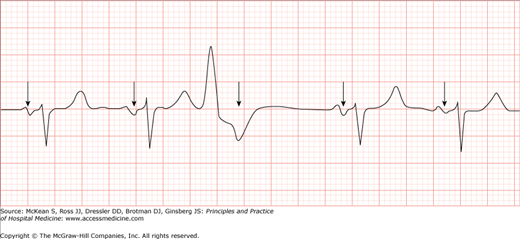
A PVC is characterized on the surface ECG by a wide (>120 msec) and bizarre QRS that is premature and is commonly followed by a fully compensatory pause (Figure 128-2). The T wave that follows a PVC will usually have a vector that is directed opposite that of the mean PVC-QRS vector. These characteristics have notable exceptions. The fully compensatory pause that follows PVCs depends on the PVC not influencing atrial depolarization or AV conduction. In most cases, PVCs will not conduct retrograde to the atria and thus will have no effect on sinus impulse formation. When these PVCs occur, the sinus impulse that happens during the PVC will not be able to conduct antegradely to the ventricles because the ventricle is refractory. However, because the sinus node is not reset by the PVC, the sinus beat following the PVC will come at a predictable time that is usually equal to twice the basic R-R interval (Figure 128-2), and usually described as a fully compensatory pause.
Figure 128-2
A typical premature ventricular complex (PVC) (third QRS from left to right). Sinus activity (arrows) is usually not disrupted by the PVC. The beat following the PVC will come at a predictable moment that is equal to twice the basic R-R interval (fully compensatory pause). (Reproduced, with permission, from Tintinalli JE, Stapczynski JS, Cline DM, et al. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York: McGraw-Hill; 2011. Fig. 22-23B.)
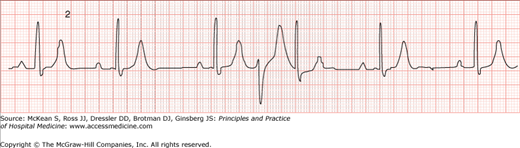
Occasionally, PVCs will have no influence on the subsequent sinus impulse and propagation. These premature beats are termed interpolated PVCs (Figure 128-3). Although PVCs with fully compensatory pauses are most common, sometimes PVCs are conducted retrograde and may result in atrial activation. This will manifest on the ECG as inverted P waves in the inferior leads after the PVC. If the sinus node is reset by this retrograde atrial activation, the pause that follows the PVC will be less than compensatory. In other cases, the PVC may not conduct to the atria but may be blocked retrograde in the AV node, rendering the node refractory. This can result in prolongation of the PR interval for the sinus beat following the PVC or even block of the next sinus P wave. The consequence of both these situations is a more than compensatory pause. Finally, with regard to the T wave vector, the usual relationship between the PVC-QRS and its subsequent T wave may be altered if there is a preexisting repolarization abnormality in the myocardium where the PVC is being originated.
Currently no data support suppressive treatment of PVCs in asymptomatic healthy individuals. The evidence describing long-term mortality risk in healthy patients with PVCs is conflicting. These patients should be reassured and offered standard cardiac risk factor evaluation and modification. The clinician should also thoroughly investigate possible exacerbating factors such as current medications (eg, stimulants), possible comorbidities (eg, hyperthyroidism), and electrolyte abnormalities.
When PVCs are clearly symptomatic, intervention should also be aimed at reducing anxiety and identifying precipitating factors, but consideration can be given to suppression of the PVCs with antiarrhythmic agents. The decision to treat with antiarrhythmics, however, must be carefully balanced. While most antiarrhythmic agents are effective at decreasing PVCs, many of these agents have known proarrhythmic properties that may render the net outcome as harm rather than benefit. As an example, in the Cardiac Arrhythmia Suppression Trials (CAST I and II), patients with a prior myocardial infarction and frequent PVCs were randomized to receive placebo versus one of three antiarrhythmic agents (flecainide, encainide, or moricizine). Although antiarrhythmic therapy proved efficacious in suppressing PVCs, the study was discontinued after 2 years’ follow-up because of an increase in overall mortality in the group that was randomized to antiarrhythmic agents. Similarly, more recent trials using amiodarone for suppression of PVCs in the setting of post-MI patients and heart failure patients have shown antiarrhythmic therapy to be effective at reducing ventricular ectopy and arrhythmic mortality but have failed to demonstrate an effect on overall mortality. Based on the available evidence, even if PVCs may have some prognostic value in the setting of certain specific cardiac conditions, there is no indication to treat PVCs in the presence or absence of heart disease to reduce mortality risk. Nonetheless, the presence of structural heart disease may warrant therapy with PVC-suppressing agents, such as beta-blockers, even when the primary aim is not to treat the PVCs (ie, beta-blockers in heart failure and post-MI).
|
If PVCs do warrant therapy based on clinically significant symptoms, beta-blockers are first-line therapy. This class of drug is particularly useful for daytime PVCs or PVCs that are related to stress, thyrotoxicosis, and mitral valve prolapse. The lowest possible dose that alleviates symptoms should be used. Other pharmacological alternatives include amiodarone and sotalol, but referral to a cardiologist is generally warranted before initiation of these latter medications. The 2006 ACC/AHA/ESC guidelines for management of patients with ventricular arrhythmias recommend radiofrequency ablation for PVCs (class IIa indication) if PVCs are frequent, symptomatic, and have proven refractory to medical therapy and if the patient is otherwise at low risk for sudden cardiac death (ie, does not meet criteria for device therapy).
Ventricular Tachycardia
|
VT is another common form of ventricular arrhythmia that has a variety of different presentations. In the most severe of cases, VT may precipitate cardiac collapse and cause SCD. SCD, commonly defined as death from an arrhythmic cause occurring within 1 hour of the onset of symptoms, accounts for up to 460,000 deaths annually in the United States. VT and VF lead to more than 80% of monitored cases of SCD. Although VT may occur in the setting of structurally and genetically normal hearts, most patients with potentially malignant ventricular arrhythmias will have some form of underlying heart disease. As an example, approximately 70% of patients with SCD will have severe coronary disease. Furthermore, over 50% of patients treated for recurrent VT will have ischemic heart disease, and most others will have some form of cardiomyopathy. The prognosis of VT largely depends on the presence of underlying heart disease. VT in the presence of structural heart disease usually confers a high risk of SCD, whereas VT in the absence of structural heart disease or inherited sudden cardiac death syndromes generally has a good prognosis.
|
In VT, symptoms are nonspecific and depend largely on the degree of hemodynamic compromise that occurs—determined by the duration of the arrhythmia, the ventricular rate, and the presence of underlying heart disease. Palpitations, heart failure, symptomatic hypotension or hemodynamic collapse, and SCD may all be presenting features of VT. VT may occur with minimal hemodynamic consequences, and the absence of symptoms and hemodynamic instability does not necessarily favor the diagnosis of another type of arrhythmia (ie, supraventricular tachycardia with aberrancy). Specific physical exam findings, as occurs with PVCs, include cannon A waves (secondary to AV dissociation), first heart sound variability, and split-second heart sounds.
The mechanisms responsible for the generation of VT are identical to those discussed for tachyarrhythmias and PVCs (ie, reentry, increased automaticity, or triggered activity) and vary according to the clinical context in which they are encountered (ie, normal heart, long QT interval, ischemic heart disease).
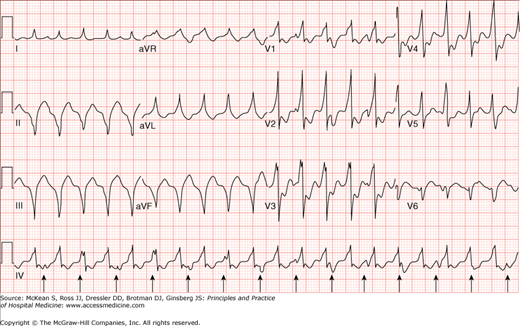
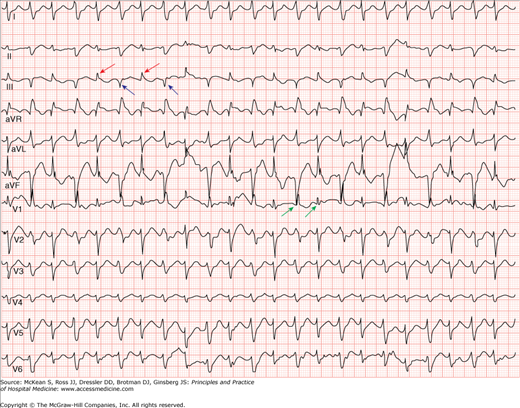
VT is defined as the occurrence of three or more consecutive ventricular beats at a rate of more than 100 beats per minute (bpm). Although ventricular beats typically originate in ventricular muscle, they can also be initiated anywhere in the specialized conduction tissue distal to the bifurcation of the His bundle. Because of its ventricular origin, the QRS in VT is usually wide (> 120 msec), bizarre, and has a T-wave vector opposite the main QRS deflection (Figure 128-4). Rarely, however, ventricular beats originating in the basal septum or high in the fascicular system may produce a narrow complex QRS (less than 5% of all VTs). VT is usually regular but slight R-R variability may exist. The rate varies anywhere from 60 to 250 bpm depending on the specific type of VT.
Figure 128-4
Ventricular tachycardia. Electrocardiogram showing a wide complex tachycardia with independent atrial activity (arrows). Atrioventricular dissociation (seen here), although not always present, is very specific for a tachycardia of ventricular origin. (Reproduced, with permission, from Fauci AS, Braunwald E, Kasper DL, et al. Harrison’s Principles of Internal Medicine. 17th ed. New York: McGraw-Hill; 2008, Fig. 226-10.)
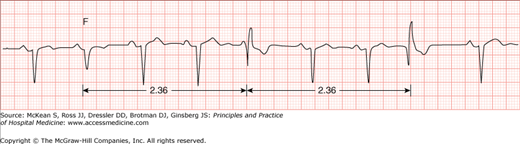
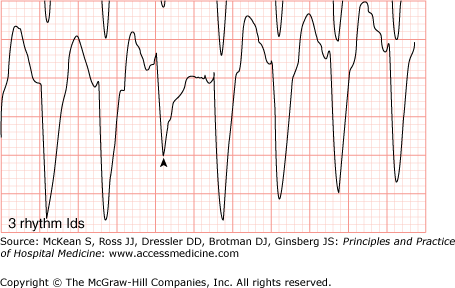
To classify VT, the morphology of consecutive beats and the duration of the VT episode are considered. The morphology of consecutive ventricular beats may be close to identical (ie, monomorphic VT) or may vary from beat to beat (polymorphic VT). Occasionally, the QRS axis will alternate predictably from beat to beat, a VT that is known as bidirectional VT (Figure 128-5), usually seen in the setting of digitalis toxicity, cardiomyopathy, or catecholaminergic polymorphic VT. The duration of VT also helps classify the tachycardia as nonsustained (arbitrarily defined as lasting less than 30 seconds), or sustained (lasting more than 30 seconds or requiring termination due to hemodynamic collapse).
Figure 128-5
Bidirectional ventricular tachycardia. Wide QRS tachycardia with relatively narrow QRS complex with right bundle branch block (RBBB) (green arrows) morphology and alternating inferior (red arrows) and superior (blue arrows) axes. (Reproduced, with permission, from Knoop KJ, Stack LB, Storrow AB, et al. Atlas of Emergency Medicine. 3rd ed. New York: McGraw-Hill; 2009. Fig. 23-32C. Photo contributor: Marc Mickiewicz, MD.)
|
The differential diagnosis of wide complex tachycardias (WCT) includes three major categories: VT, supraventricular tachycardia (SVT) with aberrant conduction, and SVT with antegrade conduction through an accessory pathway (ie, preexcitation). Though a clear distinction is often difficult to make, several clues support a diagnosis of VT in the setting of a WCT (Table 128-1). The most important clinical predictor of VT is the presence of structural heart disease. As such, a patient with a reduced ejection fraction or previous MI and a wide complex tachycardia should always be presumed to have VT (over SVT with aberrancy or preexcitation) unless there is very compelling evidence otherwise.
|
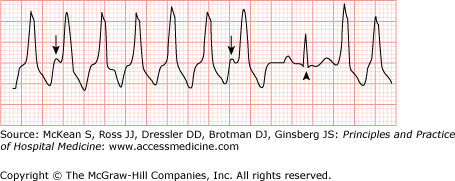
In addition, careful observation of the ECG tracing for evidence of fusion beats, capture beats, or atrioventricular (AV) dissociation will provide important clues for the diagnosis. Fusion and capture beats (Figures 128-6 and 128-7) imply activation of the ventricle from two different electrical sites during the tachycardia. Because both fusion and capture beats result in a QRS that is narrower than the QRS seen during the rest of the tachycardia, they imply intermittent partial (fusion) or complete (capture) conduction to the ventricles from a supraventricular focus and thus rule out a supraventricular origin for the tachycardia. Although evidence of AV dissociation during a WCT has long been considered a forceful argument in favor of VT, retrograde activation of the atria (ie, ventricular to atrial conduction) is seen in up to 25% of VT. Thus identification of independent atrial activity and AV dissociation during a WCT is helpful in identifying VT, but these conditions are not always present (see Figures 128-4 and 128-7), and if present they may represent retrograde conduction. If the patient is stable and there is any doubt that the rhythm is VT versus SVT, immediate expert consultation should be sought.
Figure 128-6
Fusion beat. The QRS that is highlighted by the arrowhead represents fusion of a QRS originated from both a supraventricular focus and the ventricles. (Electrocardiogram contributor: Marc Mickiewicz, MD.) (Reproduced, with permission, from Knoop KJ, Stack LB, Storrow AB, et al. Atlas of Emergency Medicine. 3rd ed. New York: McGraw-Hill, 2009. Fig. 23-32B. Photo contributor: Marc Mickiewicz, MD.)
Figure 128-7
Capture beat. Ventricular tachycardia (VT) with atrioventricular dissociation (arrows show P waves). A capture beat occurs following a lapse in the VT (arrowhead). Reproduced, with permission, from Knoop KJ, Stack LB, Storrow AB, et al. Atlas of Emergency Medicine. 3rd ed. New York: McGraw-Hill, 2009. Fig. 23-33B.)
|
The initial management of suspected ventricular rhythm disturbances requires careful assessment of stability. Stable patients bear no significant hemodynamic consequences related to the arrhythmia. Treatment of significant hemodynamic consequences (eg, extreme hypotension) or other symptoms related to hypoperfusion (ie, angina, heart failure, altered mental status) in the setting of a suspected ventricular arrhythmia should occur emergently (ie, within seconds to minutes), precluding any further diagnostic maneuvers. The diagnostic exercise of distinguishing VT from SVT with aberrancy or preexcitation by using historical, clinical, and ECG data should only be undertaken in stable patients. Synchronized cardioversion (CV) with 100–200 J (monophasic) or 50–100 J (biphasic) is the treatment of choice in patients who are unstable as a result of VT. Required knowledge and practice of both basic life support (BLS) process and algorithms and advanced cardiac life support (ACLS) process and algorithms aid successful resuscitative measures in unstable ventricular arrhythmias.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree