1. Development of the vascular system starts in early embryogenesis.
• Mesenchymal cells develop into hemangioblasts, some of which become angioblasts.
• The formation of de novo vessels is referred to as vasculogenesis; remodeling of these vessels and stromal cell recruitment is called angiogenesis.
• Lymphangiogenesis commences slightly later in development with budding from developing veins.
• Disruptions in any of these processes can lead to embryonic lethality at worst or vascular anomalies if the embryo survives.
• Anomalies can be limited to a single vessel type or involve multiple vessel types and other tissues.
2. Vascular lesions are divided into two broad classes.
• Tumors such as hemangioma are characterized by hypercellularity during the proliferative phase and then a transition to decreased cellularity with increased fibrosis during the involution phase.
• Malformations demonstrate growth commensurate with the patient’s growth and a normal rate of endothelial cell turnover.
• Identification and treatment of vascular lesions are complex, and patients with puzzling vascular lesions should be evaluated and treated at a multidisciplinary center with experience caring for vascular anomalies.
3. Hemangiomas are the most common benign vascular tumor, and are the most common soft tissue tumor in children.
• The usual clinical course is rapid proliferation for a few months, followed by regression that can take several years. The primary therapy is medical. Surgical intervention is rarely necessary unless vital structures are threatened.
• Cervicofacial hemangiomas warrant investigation for subglottic lesions.
• Multifocal hemangiomas warrant investigation for visceral/intracranial lesions.
• Diffuse hepatic hemangiomas are associated with profound hypothyroidism.
4. Vascular malformations are physiologically divided into fast-flow (arteriovenous malformations [AVMs]) and slow-flow (all other vessel types).
• They exhibit expansion over a patient’s lifetime, with accelerated expansion at puberty.
• Fast-flow lesions can result in high-output cardiac failure, pain, bleeding, and destruction of surrounding structures.
• Slow-flow lesions can result in pain, bleeding, infection, and impingement on nearby structures.
• Psychological distress and social nonacceptance are significant factors in many patients’ wish for treatment.
• Complex lesions involving multiple vessel types can be associated with problems due to limb overgrowth, scoliosis, joint involvement, and contractures.
• Lesions with stagnant blood flow due to ectatic vessels can cause a consumptive coagulopathy and an elevated risk of thromboembolism in the operating room (OR) and in daily life.
• Disorders of the central conducting lymphatics result in dysfunctional lymphatics, causing leakage and fluid accumulation in nearby potential spaces. Ascites and pleural or pericardial effusions can make anesthetic management problematic.
• Treatment is recommended earlier in life, when lesions are smaller.
• Medical therapy is an evolving field, with promising medications being aimed at various molecular targets in the angiogenic and lymphangiogenic pathways.
• First-line treatment for most lesions is in the interventional radiology (IR) suite, with surgery reserved for lesions that are either small and easily removed or no longer responsive to sclerotherapy/embolization.
• Consideration must always be given to the potential morbidity and scarring involved in a large surgical resection.
5. The majority of interventions/anesthetics for these patients at our institution occur in the IR suite rather than in the OR.
• Anesthesia outside the OR is associated with increased risk.
• Provider protection from radiation must also be assured.
• An IR suite is designed to reduce radiation exposure for the radiologist.
• Scatter radiation causes anesthesiologists to incur four times the dose that the radiologist does while at the same distance from the patient on the procedure table.
• The basic elements of radiation protection can be summarized as increased shielding, decreased time of exposure, and increased distance from the radiation source.
6. Anesthesia equipment for the IR suite should be standardized to those used in the main ORs.
• Advanced airway equipment should be readily available for patients with malformations near the airway.
7. Patient preparation may require expertise from multiple disciplines to optimize comorbid conditions prior to an anesthetic.
• Our experience mandates hematologist input for perioperative anticoagulation and possibly inferior vena cava (IVC) filter placement for all patients with complex vascular anomalies.
• Patients with anomalies near the airway should be evaluated by an otorhinolaryngologist to determine whether they will present an airway management challenge.
• Patients with high-output cardiac failure will need medical optimization of heart failure.
8. Cases in IR or the OR can vary in length from an hour to 8 to 10 hours. Most cases will require general anesthesia, with airway management choices predicated on patient position and comorbidities and expected procedure length.
• Regional techniques may be contraindicated due to the relationship of the lesion to target nerves, or coagulopathy.
• IR cases are unlikely to involve blood loss. Surgical cases can involve massive blood loss, and preparations for massive transfusion should be in place prior to the procedure when warranted.
9. Postoperative care of patients after massive resection may be complicated by a patient’s chronic pain. Pain service input may be necessary.
• Swelling after sclerotherapy does not peak until several hours postprocedure. Care must be taken when considering extubation after treatment of lesions near the airway. Intubation may be necessary for several days.
• Pain is agent-dependent after sclerotherapy and can be significant.
I. Introduction
A. Errors in the development of the vascular and lymphatic systems result in abnormalities with purely cosmetic implications to massive malformations involving multiple vessel types and many associated comorbidities.
B. Vascular anomalies affect approximately 4% to 10% of the population (1) and, with the exception of infantile hemangioma, usually present in childhood or adolescence.
C. Patients with vascular anomalies, and particularly those with complex lesions, may have suffered through years of misdiagnosis and suboptimal treatment. A proper understanding of the origin, natural history, comorbidities, and appropriate treatment of these disorders is crucial to providing care to this challenging patient population.
D. This chapter will review the development, classification, and natural history of vascular anomalies, the physiologic considerations associated with various malformations, and some specific procedures performed on these patients, with a discussion of their anesthetic concerns.
II. Development of the vascular and lymphatic systems
A. The vascular system’s network of blood and lymphatic vessels is crucial for transporting gases, nutrients, waste products, signaling molecules, immune cells, and liquid around the organism once an embryo has become too large for passive diffusion of these substances.
1. Errors at any one of numerous stages in this complex process, if not lethal to the organism, can result in vascular anomalies. A number of these processes are now being described at the molecular level.
B. Formation of the cardiovascular system’s capillaries, arteries, and veins as the result of a cascade of molecular signaling actions is one of the earliest events in embryogenesis, commencing around the third week of development in humans (2–4).
1. At the time of gastrulation, mesenchymal cells differentiate into hemangioblasts, bipotential cells that give rise to both blood and endothelial cells (Fig. 19.1).
2. Hemangioblasts aggregate into blood islands and complete their differentiation into blood cells (angioblasts), the precursor to endothelial cells.
3. Endothelial cells then fuse to form primary capillary plexuses. This process of de novo differentiation, migration, and aggregation of mesodermal precursors is referred to as vasculogenesis (2).
4. Capillary plexuses then experience a process of remodeling, pruning, lumen formation, support cell recruitment, and sprouting events that results in a network of capillary beds and major vessels in the embryo. This process of forming blood vessels from preexisting structures is referred to as angiogenesis.
C. Several of the crucial signaling pathways involved in vasculogenesis and angiogenesis have been described, and are treated in detail in references (3) and (5–7).
1. Several signaling systems utilize the various vascular endothelial growth factors (VEGFs) and their receptors.
D. Another system which is both extensively utilized in vasculogenesis and well preserved evolutionarily is the Notch/Delta receptor/ligand complex (8).
E. Patterning of vessels within the developing organism such that the aorta and major draining veins form in the dorsal midline relies on VEGF gradients from the definitive endoderm that attracts angioblasts to their correct position in the embryo (9).
F. While capillaries consist of a single layer of endothelial cells, other vasculature consists of layers of endothelial cells, smooth muscle cells, and various other stromal cells, referred to as pericytes.
G. Lymphatic vessels consist of blind-ended capillaries with discontinuous junctions between endothelial cells and sparse basement membrane.
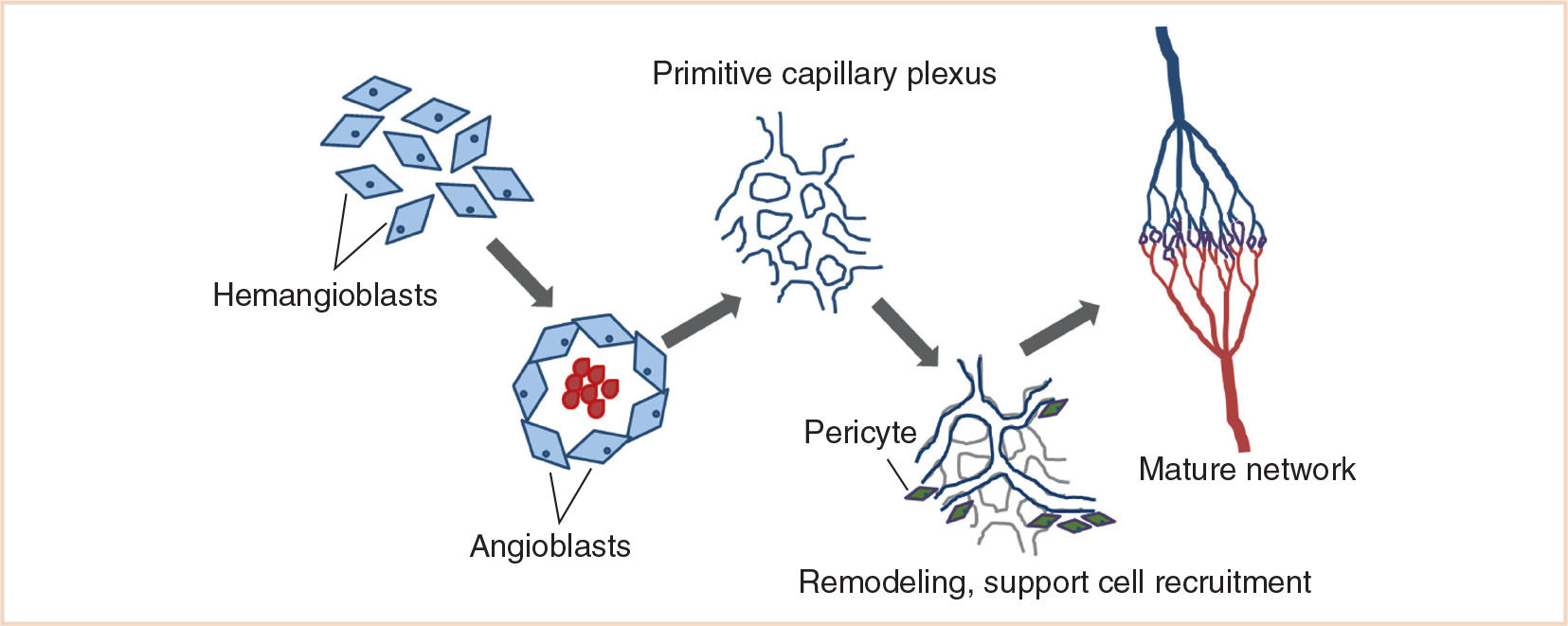
FIGURE 19.1 Outline of the stages of vasculogenesis and angiogenesis. (Adapted from Boon LM, Vikkula M. Molecular genetics of vascular malformations. In: Mulliken JB, Burrows PE, Fishman SJ, eds. Mulliken and Young’s Vascular Anomalies: Hemangiomas and Malformations. 2nd ed. Oxford, UK: Oxford University Press; 2013:327–375.)
1. Lymphatic capillaries drain into larger collecting vessels that are valved and are lined by smooth muscle cells that contract to regulate lymph flow.
2. Other than connections at the subclavian veins, the lymphatic and blood systems do not communicate.
H. Lymphangiogenesis begins slightly later in development, around the 6th to 7th week of human embryonic development (10,11).
1. Once the major dorsal vein has developed, a subpopulation of endothelial cells commits to the lymphatic lineage and buds from the vein to form primordial lymph vessels (referred to as “lymph sacs” in early literature) (12).
2. The primitive lymph vessels remodel and sprout, forming a system of lymph capillaries and collecting vessels, with the latter developing valves and attracting pericytes.
I. The connection between the events of vasculogenesis, angiogenesis, and lymphangiogenesis with the development of vascular anomalies is very apparent and is being actively unraveled at a molecular and developmental level. While it is well beyond the scope of this chapter to describe in detail the molecular causes of the various vascular anomalies and their ties to normal vasculogenesis, several excellent reviews cover this subject matter in depth (13–15).
III. General classification of anomalies
A. Historically the classification of vascular lesions has been hampered by several factors:
1. Many of these lesions superficially resemble each other.
2. Many types of anomalies exist, most of which are quite rare.
3. Reliance on an admixture of descriptive and histologic terms has clouded clear communication in the field (16,17).
B. For example, the term “hemangioma” has been applied to lesions ranging from the classic involuting hemangioma of infancy to extensive intramuscular venous malformations, lesions with very different causes and clinical courses (18).
1. Given the variety of biologic processes underlying the spectrum of vascular lesions, therapy that may be both appropriate and effective for one lesion may well be not for a lesion of a different type.
a. 47% of patients referred to the Boston Children’s Hospital Vascular Anomalies Center had an incorrect referral diagnosis.
CLINICAL PEARL Patients with puzzling vascular lesions should be evaluated and treated at a multidisciplinary center with experience in the care of this population in order to reduce wasted effort and potential harm to patients with inappropriate therapies (19).
C. In 1982, Mulliken and Glowacki proposed a basic subdivision of vascular lesions into either tumors or malformations (17).
1. Tumors (the most common being hemangiomas) were characterized by hypercellularity during the proliferative phase and then a transition to decreased cellularity with increased fibrosis during the involution phase.
2. On the other hand, lesions present at birth that demonstrated growth commensurate with the patient’s growth and a normal rate of endothelial cell turnover were classified collectively as vascular malformations.
3. This basic subdivision has proven to be a useful guide for the diagnosis and clinical management of vascular lesions.
4. It has been updated several times over the years to reflect expanding knowledge, and is now referred to as the ISSVA classification (20).
D. Tumors
1. Infantile hemangioma
a. Infantile hemangiomas are the most common benign vascular tumor, and are the most common soft tissue tumor in children (21).
b. As swellings with or without color change of overlying skin, they are typically not present at birth, although recent data indicate that in 65% of patients a precursor skin mark is present (22).
c. The majority of hemangiomas present as isolated lesions, but 20% present as multiple lesions, and are more strongly associated with visceral or intracranial hemangiomas. (23,24).
d. Their usual clinical course is rapid proliferation for a few months followed by regression that can take several years.
e. Most of these lesions involute without problem and do not require treatment.
f. For the minority of infantile hemangiomas that do require treatment, it is usually because of disfigurement or mass effect threatening vital structures such as the airway or the eye.
(1) Cervicofacial hemangiomas, particularly those in a “beard” distribution, warrant investigation for the presence of subglottic hemangioma (25), although airway hemangiomas can occur in the absence of any cutaneous signs.
(2) Segmental hemangiomas, which involve an anatomic territory, have been associated with other congenital problems (Posterior fossa malformations-hemangiomas-arterial anomalies-cardiac defects-eye abnormalities (PHACE), Lower body hemangioma, Urogenital anomalies, Myelopathy, Bony deformities, Anorectal malformations, Arterial anomalies, and Renal anomalies (LUMBAR) associations), including cardiac anomalies (26).
g. The primary therapy for hemangiomas, even subglottic, is medical, with propranolol having taken the place of systemic steroids as the first-line treatment (27).
(1) It is uncommon for these lesions to require surgery in the proliferative phase unless medical therapy has failed, although cosmetic surgery to improve the appearance of an involuted hemangioma in an older child may occur (28).
2. Congenital hemangioma
a. A rare subtype, congenital hemangioma, is fully formed at birth and, on occasion, is identified prenatally (29).
b. They either involute rapidly by the first year of life or fail to involute.
c. Rapidly involuting congenital hemangiomas (RICH) as well as noninvoluting congenital hemangiomas (NICH) can be associated with transient coagulopathy, with thrombocytopenia, and with arteriovenous shunting significant enough to cause cardiac compromise (30,31).
d. The presence of shunting with cardiac compromise may warrant embolization of feeder vessels to the lesion.
3. Hepatic hemangioma
a. Hepatic hemangiomas can be separated into focal and diffuse lesions.
b. Focal hepatic hemangioma is the hepatic counterpart of RICH (32).
(1) High-output heart failure from arteriovenous shunting within these lesions has been described, as has thrombocytopenia and abdominal compartment syndrome.
(2) Regression of the lesion usually occurs within the first year of life.
c. Multifocal and diffuse hepatic hemangiomas have a life cycle more typical of infantile hemangioma (24).
(1) Multifocal hemangioma can cause high-output cardiac failure, while diffuse hepatic hemangioma generally does not.
(2) Diffuse hepatic hemangioma can result in hepatic failure due to extensive replacement of hepatic parenchyma.
(3) Diffuse (and some multifocal) hepatic hemangiomas will result in profound hypothyroidism due to lesional expression of type 3 iodothyronine deiodinase (33)—this can be quite resistant to treatment and needs close monitoring to assure adequate replacement.
(4) Medical therapy with propranolol or systemic steroids has been reported to have some benefit.
(5) Intra-arterial embolization has been used in cases where patients are experiencing high-output heart failure. Surgical resection is utilized less often.
(6) Surgery is generally unnecessary in the case of one or even several small lesions, and more diffuse presentations are not amenable to resection.
(7) Liver transplant has been described in a few cases (24,28,34).
(8) Anesthetically these cases are extraordinarily challenging due to the likely presence of heart failure and massive abdominal distention in a very young infant.
E. Malformations
• Vascular malformations are classified based on the vascular tissue or tissues from which they arise.
• Physiologically they can be divided into fast-flow (arteriovenous) and slow-flow (venous, lymphatic, capillary, and most mixed) lesions, with problems particular to each.
• As a class, vascular malformations are far less common than vascular tumors, affecting approximately 1:200 people compared to 1:20 (19).
1. Capillary malformations
a. Capillary malformations (CMs) are the most common and generally the most benign vascular malformation. The majority of CMs are isolated and have no association with any other abnormalities. A small number are associated with an underlying syndrome.
b. Up to 40% of infants have a fading capillary stain (nevus simplex) present at birth, either on the forehead or on the nape of the neck. They are distinct from true CM and are not associated with Sturge–Weber syndrome. Treatment is rarely needed for these lesions, although pulsed dye laser treatment of persistent stains in cosmetically sensitive locations may be utilized.
c. True CMs (nevus flammeus) occur in approximately 0.3% of all infants, and consist of a network of initially histologically normal capillaries with telangiectasias and vascular ectasias developing over time (35).
d. These lesions are present at birth and grow proportionally with the affected body part, generally darkening and becoming thicker over time. Hypertrophy of underlying soft tissue and bone is not uncommon as time progresses.
(1) The majority of CMs will not require treatment unless they are in a cosmetically significant area such as the face.
(2) The mainstay of therapy is a course of pulsed dye laser therapy, which may require several general anesthetics for younger children or for more extensive lesions.
e. There is no data showing that early treatment of CM will reduce the incidence of problems such as thickening of the CM or overgrowth of underlying tissue (36), but the psychological benefits of early therapy of CM for a child’s developing self-image have been described (37).
f. Medical therapy for many vascular malformations, including CM, has focused on antiangiogenic therapy to slow the growth and progression of the lesion, although studies of efficacy are still ongoing (35).
g. 6% to 10% of patients with CM in the facial V1 dermatome have Sturge–Weber syndrome, which comprises facial CM, ipsilateral leptomeningeal vascular malformation, and choroidal vascular malformation of the eye. These patients can manifest seizures, developmental delay, hemiparesis, and glaucoma (38).
(1) Facial hemihypertrophy with overgrowth of soft tissue (lip, cheek, or forehead) and bony structures (maxilla, mandible) is present in up to 60% of patients, many of whom will require surgery for cosmetic and functional improvement (39).
h. Other syndromes associated with CM are quite rare.
(1) Macrocephaly–capillary malformation (M-CM) (formerly M-CMTC) is characterized by CM with macrocephaly, hypotonia, developmental delay, and skin laxity. In some cases, hemihypertrophy is present (40,41).
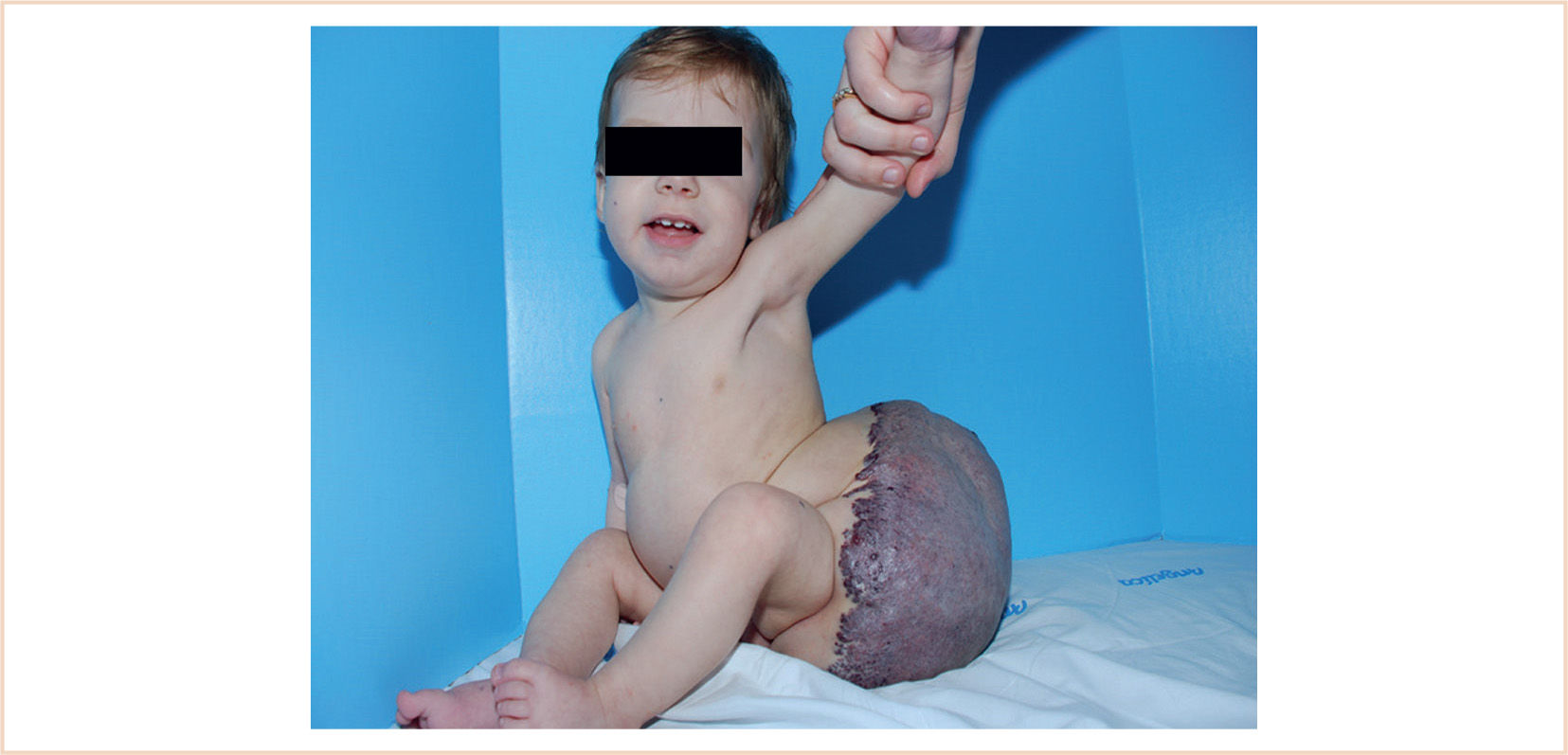
FIGURE 19.2 A 2-year-old boy with blue rubber bleb nevus syndrome and massive venous malformation of left thigh. Some characteristic indigo nevi are apparent on his chest and cheek.
(2) Diffuse capillary malformation with overgrowth has more recently been described and differentiated from other syndromes of CM with overgrowth such as Klippel–Trénaunay and CLOVES (congenital, lipomatous overgrowth, vascular malformations, epidermal nevi and spinal anomalies) (described later) (42).
(3) Capillary malformation–arteriovenous malformation (CM-AVM) is an autosomal dominant condition in which patients have both small multifocal CM and underlying AVM.
2. Venous malformations
a. Venous malformations (VMs) are characterized by dilated, thin-walled venous channels surrounded by abnormal smooth muscle (Fig. 19.2).
b. Although present at birth, VMs may not come to clinical attention until later in childhood when they have grown sufficiently to be visible or cause symptoms.
c. 90% of VMs are sporadic and solitary (43).
d. Half of all VMs are confined to the subcutaneous tissue, while the rest also affect muscle, bone, joints, or viscera.
e. Ten percent of patients with VM have multifocal inherited lesions such as glomuvenous malformation, caused by an autosomal dominant mutation in the glomulin gene (44).
f. Other inherited VM conditions include cutaneomucosal VM and cerebral cavernous malformation, the latter of which increases the risk for intracranial hemorrhage (45).
g. Blue rubber bleb nevus syndrome (BRBNS), characterized by multifocal VMs both cutaneous and throughout the gastrointestinal (GI) tract, has been described as having both familial and sporadic causation (46).
h. Over time, vascular channels expand in the VM, resulting in a number of indications for treatment.
(1) Psychological distress on the part of either the patient or their family at the appearance of a malformation is not uncommon (47).
(2) Pain and swelling are the most common complaints.
(a) Swelling of the VM itself can be painful, particularly if it is within a tissue compartment that does not allow for limitless swelling.
(b) Intralesional thrombi, which are a result of flow stagnation within the lesion, can also be a significant source of pain.
i. Flow stagnation and ongoing thrombosis within a VM can result in consumptive coagulopathy.
CLINICAL PEARL Lesions with stagnant blood flow due to ectatic vessels can cause a consumptive coagulopathy and an elevated risk of thromboembolism in the operating room (OR) and in daily life. A hematologist familiar with this condition should be consulted for periprocedure management.
(1) This has been referred to as LIC (localized intravascular coagulopathy) and is characterized by numerous coagulation profile anomalies, including low fibrinogen and elevated D-dimer (48). Thromboembolism from a sporadic VM is less likely, as these are usually sequestered from the deep circulation (43).
(2) Patients with extensive VMs which involve the deep venous system as well as patients with syndromes which involve venous ectasia and stasis, such as Klippel–Trénaunay and CLOVES, are at increased risk of chronic small pulmonary emboli leading to elevated right-sided cardiac pressures (49) and life-threatening thromboembolism (50,51). Care must be taken in the perioperative period to manage LIC so that patients do not deteriorate into either disseminated coagulopathy or uncontrolled thrombosis/thromboembolism. Counterintuitively, LIC is managed by anticoagulation, stopping clot formation in the VM, and releasing more clotting factors to the rest of the organism.
(a) This should be initiated and monitored by hematologists experienced in the care of patients with vascular anomalies and, depending on the patient, may be necessary either lifelong or only periprocedurally (48).
j. The natural history of VMs is to expand over time. While vascular malformations as a rule grow with the patient during childhood, they have a high likelihood of faster growth during adolescence.
(1) Overall, a patient with a VM has a 75% chance of its expanding or becoming symptomatic by adulthood (52).
(2) Expansion during adolescence may be due to the presence of progesterone receptors in VM (53). Estrogen and testosterone have been shown to directly stimulate VEGF production (52).
k. Current thinking supports treatment of asymptomatic VMs earlier in childhood, before a lesion has expanded and made treatment more challenging. In general, we wait, when possible, until at least a year of age, when anesthetic risks are somewhat decreased.
l. An expanding VM can impinge on nearby structures, particularly when the VM is in the head or neck, resulting in distortion and/or obstruction of the airway or orbits.
m. VMs can bleed through the overlying skin or mucosa. This can be particularly problematic for malformations in the oral cavity and GI tract. Bleeding from the latter can result in transfusion-dependent anemia, notably in BRBNS (46).
n. Treatment of VM is multimodal, and depends very much on the size and location of the lesion.
o. The main indications for treatment are pain, impingement on vital structures, disfigurement, and bleeding (54).
(1) Sclerotherapy is the first-line treatment of VMs, being less invasive and equally, if not more, effective as surgery (55).
(2) Good-to-excellent results were reported in 75% to 90% of patients in one series, although commonly multiple treatments are necessary for satisfactory results (56). Surgery is not favored as a first-line treatment due to the risk of significant intraoperative bleeding, iatrogenic injury to nearby structures, and postoperative disfigurement.
(3) Even when a VM can be excised surgically, it is important to consider the risk of the resulting scar or deformity (43).
(4) Resection is generally reserved for small confined lesions or for those with persistent deformity or symptoms after options for sclerotherapy have been exhausted.
p. Intra-articular VM can be particularly problematic.
(1) Over time, a mass can form within the joint space, or arthritis can develop. This results in pain, stiffness, contractures, and hemarthrosis.
(2) Joint synovectomy is used to decrease episodes of bleeding into the joint space.
(3) In extreme cases, arthropathy can render the limb nonfunctional, and joint replacement or even amputation may be necessary to restore mobility (57).
3. Lymphatic malformations
a. Lymphatic anomalies are the result of errors in the embryonic development of the lymphatic system or of functional defects in the lymphatic vessels, resulting in either discrete lymphatic malformations (LMs) or dysfunctional lymphatic vessels.
b. LMs result from faults during embryonic development of the lymphatics, resulting in lymph-filled channels with no connection to the normal lymphatics (58,59).
(1) Malformations are characterized clinically by the size of their internal channels as microcystic, macrocystic, or mixed, although histologically they are indistinguishable.
(2) LMs are benign, although similar to VMs, they do grow over the course of a patient’s life, with a tendency toward increased growth at the time of puberty (60).
(3) Treatment of asymptomatic LMs is not mandatory, although given the known tendency of these lesions to both grow and incur complications, they are often treated on presentation.
c. The most common presenting complaints for an LM are bleeding and infection.
(1) Bleeding is most commonly intralesional, resulting in pain and swelling, but frank bleeding can occur.
(2) Intralesional bleeding results from abnormal venous channels in the lesion, or occasionally from small arteries in the lesional septi, and is seen in approximately a third of LMs.
(3) While intralesional bleeding is usually small volume, it is possible for a bleeding into an infant’s large macrocyst to result in a significant loss of intravascular volume and a concomitant consumptive coagulopathy as the blood in the cyst clots.
(4) In general, full-blown coagulopathy is not observed in simple LMs (48).
d. Infection of the lymphatic fluid within malformations is seen in about 70% of LMs (61).
(1) LMs are prone to infection due to protein-rich lesional fluid and because these malformed channels are not in continuity with the lymphatic network and cannot clear out foreign material.
(a) Cutaneous vesicles are particularly problematic, serving both as a conduit for bacterial entry and as a source of drainage and bleeding.
(b) Intravenous antibiotics are often necessary, and sepsis is a very real possibility in these patients (59).
e. The other major indication for treatment is anatomic impingement on surrounding structures.
(1) LMs are more common in the lymphatic-rich area of the body, with half of them located in the head and neck (62).
(2) Large cervicofacial LMs involving the tongue, floor of mouth, neck, and mediastinum present a lifelong management challenge (Fig. 19.3).
(3) These can be diagnosed in utero (63), and on occasion warrant delivery by EXIT.
(a) In general, oral intubation is possible at delivery and a difficult peripartum tracheostomy is not required (59,64).
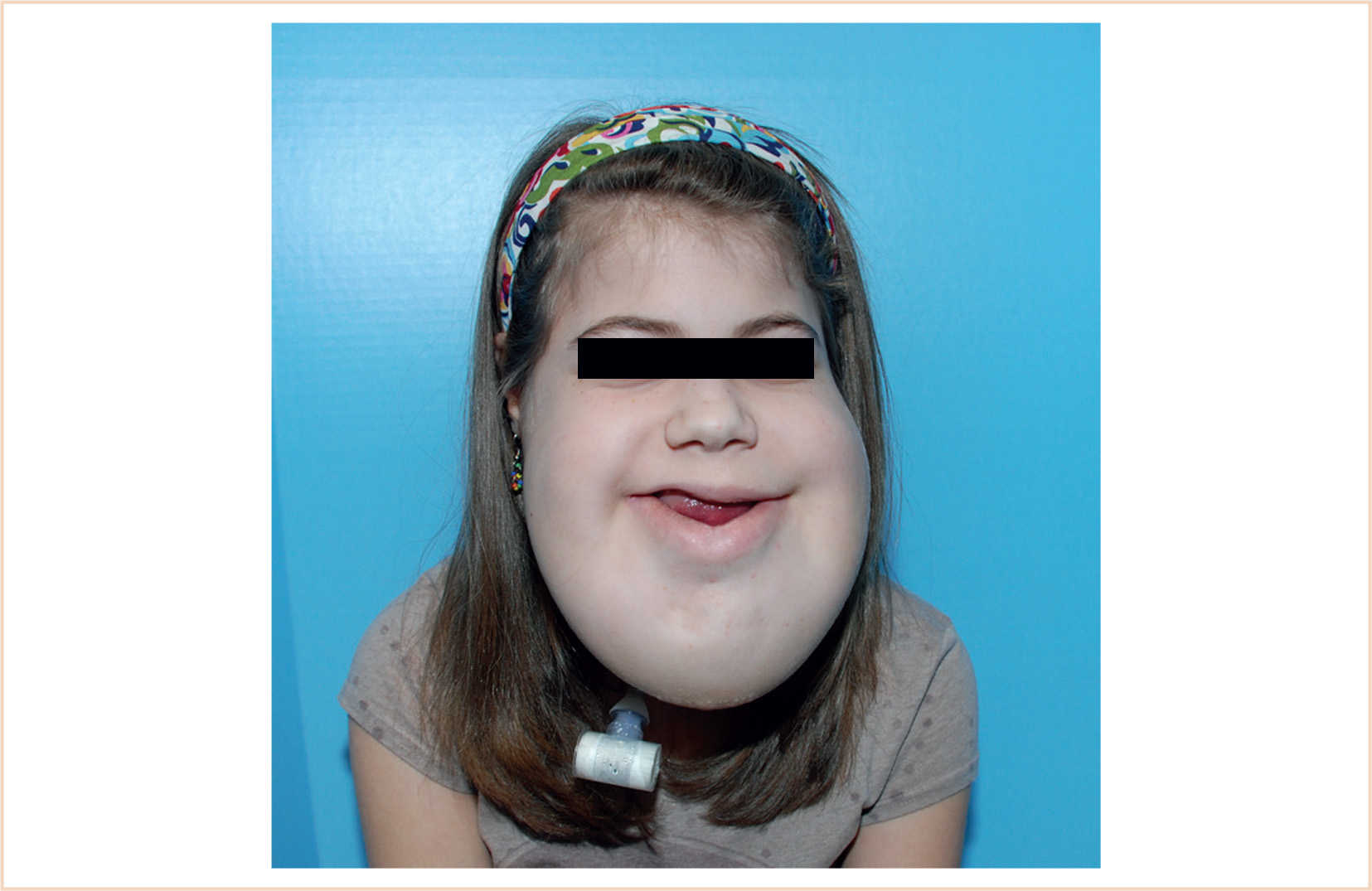
FIGURE 19.3 A 13-year-old girl with a massive cervicofacial lymphatic malformation. This patient underwent tracheostomy as an infant.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








