At room temperature and one atmosphere pressure, most anaesthetic agents exist in their liquid form. The vaporising chamber is fully saturated with anaesthetic vapour. Wicks help to increase the surface area of the anaesthetic liquid by drawing it up through capillary action. Baffles direct the vaporiser flow towards the liquid. This ensures that gas leaving the chamber is fully saturated with anaesthetic vapour and helps maintain accuracy at higher flow rates.
When gas leaves the chamber, energy is lost in the form of latent heat of vaporisation. Because SVP falls with temperature, a reduction in the operating temperature of the vaporiser would affect the concentration delivered to the patient. Heat sinks made of copper encase the vaporiser. They have a high specific heat capacity and allow the exchange of thermal energy between the device, vaporising chamber and atmosphere to minimise the temperature disruptions cause by vaporisation.
Applied science
How is the plenum vaporiser output calculated?
The SVPs of anaesthetic agents at room temperature and one atmosphere pressure are far above that required for general anaesthesia. For example, isoflurane has a SVP of 239 mmHg at 20°C, which would yield a gas concentration of (239/760 × 100) = 31.4% in the vaporising chamber. Vaporisers are designed to deliver clinically useful concentrations by altering the splitting ratio. The gas concentration in the vaporising chamber is given by the formula:
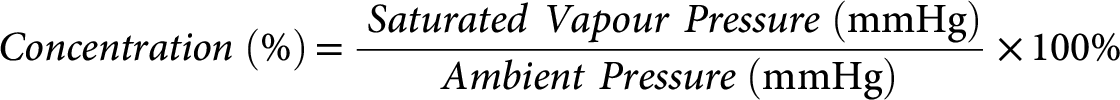
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





