Key Clinical Questions
What are the key factors that determine when to proceed to aortic valve repair, implantation, or replacement for patients with aortic stenosis? What guides decision-making relating to aortic surgery for acute aortic regurgitation?
When should patients with chronic aortic regurgitation undergo surgery?
What are the indications for surgery in patients with mitral stenosis? What are the goals of medical therapy for mitral regurgitation and when should patients be considered for mitral valve repair or replacement?
What are the objectives of treatment of pulmonic stenosis? When should a patient with pulmonic regurgitation be referred for surgery?
What are the factors that determine medical versus surgical treatment of tricuspid stenosis or tricuspid regurgitation?
Introduction
Valvular heart disease is a common disorder seen by hospitalists. Often a cardiologist and/or cardiac surgeon is consulted to comanage these patients; however, a comprehensive understanding and appropriate screening by an internist is important for both an inpatient and outpatient practice. This chapter provides practical information regarding the pathophysiology, diagnosis, and therapy of the valvular abnormalities that commonly affect each of the four valves.
Aortic Valve Disorders
Aortic stenosis (AS) is a disease of the aortic valve that causes obstruction to the ejection of blood from the left ventricle into the aorta. It is caused by three distinct abnormalities: congenital, rheumatic, and degenerative. Degenerative stenosis (also known as calcific or senile AS) is the leading cause of valvular cardiac surgery in adults. The prevalence is estimated from 2–7% in populations over the age of 65.
Calcific aortic disease represents a spectrum ranging from aortic sclerosis, defined as leaflet thickening without significant obstruction, to severe AS (defined below). Several risk factors have been associated with aortic sclerosis including smoking, hypertension, diabetes, LDL-cholesterol, and C-reactive protein (CRP). Though calcification can occur during the degeneration of all aortic valves, it tends to be more pronounced in patients with congenital abnormalities of the valve (ie, bicuspid valves) than the calcification of the trileaflet valve (the most common anatomy of AS).
Rheumatic heart disease has historically been an important cause of AS, but due to improvements in therapy and diagnosis it is uncommon in industrialized countries. Rheumatic disease remains an important cause of AS in developing nations, and should be considered in immigrant populations. Other uncommon causes of AS include connective tissue diseases, radiation therapy, and hyperlipoproteinemia syndromes. Chronic renal disease is associated with abnormal calcium homeostasis, and has been shown to hasten the progression of AS.
More recent data supports the hypothesis that aortic valve calcification is an active process that may not completely reflect patient age (and “over-use”) of the valve. Abnormalities of blood flow across the valve can lead to damage of the valvular endothelium. Endothelial injury initiates an inflammatory process similar to atherosclerosis and ultimately leads to deposition of calcium on the valve and eventual stenosis. Patients with congenitally bicuspid valves are exposed to more profound abnormalities in blood flow and develop AS earlier than trileaflet valves, usually in their fifth to sixth decade, but it can occur as early as their fourth decade. In rheumatic disease, an autoimmune inflammatory reaction is triggered by prior Streptococcus infection that targets the valvular endothelium leading to inflammation and eventual calcification.
Stenosis of the aortic valve occurs slowly and is subclinical until the disease is fairly advanced. Progressive calcium deposition and inflammation limit aortic leaflet mobility. The stenosis imposes a pressure burden on the left ventricle, which concentrically hypertrophies in response to maintain normal wall stress (afterload). With advanced disease, the adaptive mechanism fails and left ventricular wall stress increases. Systolic ventricular function declines as wall stress increases, and heart failure is inevitable in untreated disease.
|
Many of the symptoms of AS can be attributed to left ventricular hypertrophy (LVH). The consequence of concentric LVH is a smaller, less compliant chamber. Thus, left ventricular end-diastolic pressure is often increased to maintain stroke volume, especially during periods of increased cardiac output (eg, exercise), leading to the sensation of dyspnea. Furthermore, hypertrophied heart muscle requires more blood flow than normal heart muscle during exercise (supply-demand mismatch) and is subject to ischemia manifesting as chest pain even in the absence of coronary atherosclerotic disease. Areas of relatively ischemic heart muscle are potential sources of arrhythmia that can result in ventricular tachycardia and sudden cardiac death.
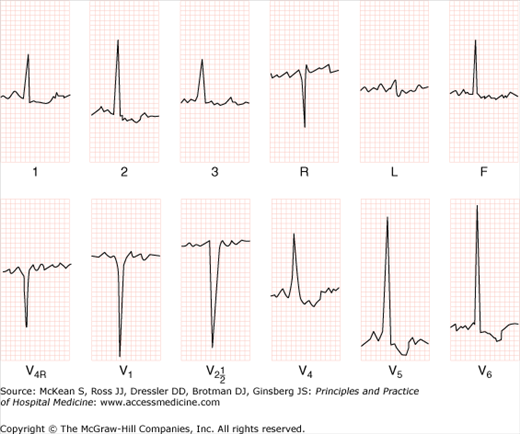
Aortic stenosis may exhibit a variety of possible clinical presentations and symptoms (Table 131-1). An ECG is indicated in the initial workup of all patients with valvular disease and is abnormal in more than 90% of patients with AS (Figure 131-1). The most common abnormality seen on the ECG is LVH due to pressure overload. Evidence of LVH and absent Q waves helps distinguish AS from other conditions such as aortic sclerosis with ischemic heart disease. Patients with AS often have conduction disease manifesting as AV block, hemiblock, or bundle branch block.
| Aortic Stenosis Symptoms | Aortic Stenosis Physical Exam Findings |
|---|---|
| Decreased exercise tolerance, shortness of breath on exertion, chest pain, syncope/near syncope, heart failure |
|
Transthoracic echocardiography is the test of choice in the evaluation of suspected AS and for the evaluation of all pathologic murmurs detected on physical exam (Figure 131-2). Echocardiography can accurately detect the presence of a pressure gradient across the aortic valve. It can also assess left ventricular function and the presence of LVH. Anatomical details of the valve stenosis can be seen and used to determine the etiology of AS. An aortic valve area of > 1.5 cm2 is mild stenosis; aortic valve area of 1.0–1.5 cm2 is moderate stenosis; aortic valve area < 1.0 cm2 is severe stenosis.
Figure 131-2
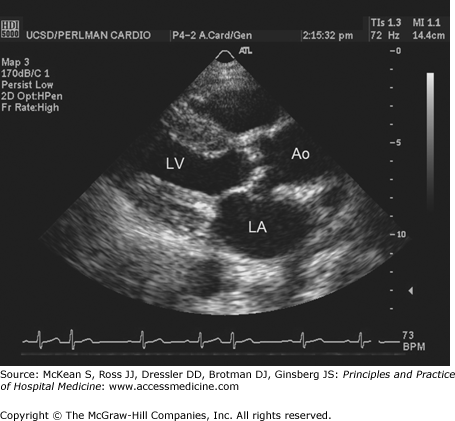
Cardiac echocardiography in the parasternal long axis view shows thickened, stenotic aortic valve and left ventricular hypertrophy. Ao, aorta; LA, left atrium; LV, left ventricle. (Reproduced, with permission, from Fuster V, O’Rourke RA, Walsh RA, et al. Hurst’s the Heart. 12th ed. New York: McGraw-Hill, 2008. Fig. 16-59.)
|
Cardiac catheterization is no longer the test of choice for the diagnosis of AS. Catheterization is used only when echo-Doppler exam is inconclusive or there is a discrepancy between noninvasive findings and the physical exam. Catheterization remains essential, however, prior to aortic valve replacement for the evaluation of coronary artery disease.
The key factors to proceed to aortic valve repair, implantation, or replacement are the onset of symptoms and left ventricular function. The onset of symptoms portends a poor prognosis with an average survival of only two to three years unless replacement is performed. Additionally, symptomatic patients are at risk of sudden death (8–34%), and thus, a strategy of “watchful waiting” should never take the place of prompt referral. Patients with failing left ventricular function and severe AS should be referred for treatment regardless of symptoms.
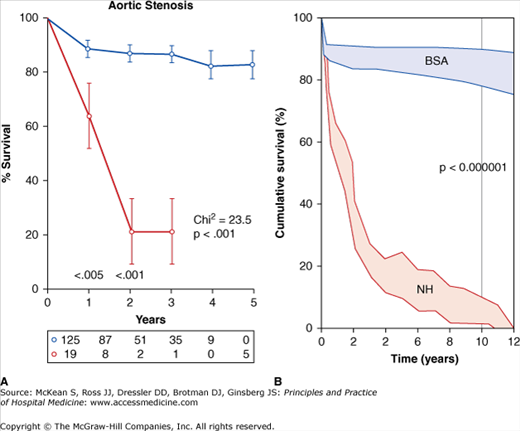
Surgical aortic valve replacement is the only therapy with proven survival benefit (Figure 131-3). A thorough discussion between the surgeon and patient exploring the risks and benefits of valve replacement should take place before proceeding with surgery. Physicians should also discuss the merits and drawbacks of the different types of valves (bioprosthetic versus mechanical) before the operation. Current bioprosthetic valves are made of porcine or bovine pericardial tissue and require reoperation 10 to 20 years after implantation; these valves do not require long-term anticoagulation. Mechanical valves are not prone to degeneration but patients are exposed to the risks of lifelong anticoagulation. Therefore the patient’s bleeding risks are very important when selecting the type of valve to implant.
Figure 131-3
Plot of survival versus time in patients with severe, symptomatic aortic stenosis that underwent surgical valve replacement (blue lines, BSA) versus best medical therapy (red lines, NH, natural history of aortic stenosis). (Reproduced, with permission, from Fuster V, O’Rourke RA, Walsh RA, et al. Hurst’s the Heart. 12th ed. New York: McGraw-Hill, 2008. Fig. 75-6.)
For patients who are highly symptomatic yet too high-risk to undergo an operation because of risk factors such as severe lung disease or porcelain aorta, balloon valvuloplasty is a reasonable option. This is a percutaneous procedure done in the cardiac catheterization lab in which a balloon is used to dilate the aortic valve and relieve stenosis and almost all patients do have symptomatic relief. Unfortunately, all patients restenose eventually at 6–12 months, some earlier and some later, and there is no improvement in mortality. Currently this technique is used for palliation in patients with no further options, or as a bridge to surgical or transcatheter aortic valve implantation.
Transcatheter aortic valve implantation (TAVI), placement of a stent-mounted bioprosthetic aortic valve using a catheter delivery system) is quickly becoming the treatment of choice in high risk or nonoperable patients. This approach avoids the morbidity of sternotomy and may be safer for elderly patients with serious and multiple comorbidities. Early experience in Europe and Canada has demonstrated the feasibility of the procedure, and follow-up, though relatively short, has been encouraging. Currently in the United States this therapy is only available through randomized trials that are currently underway to further evaluate the safety and efficacy of TAVI.
Aortic regurgitation (AR) is the diastolic reversal of blood flow from the aorta back into the left ventricle. It occurs as result of inadequate coaptation of valve leaflets due to either valve abnormalities or dilation of the aortic root. AR is a serious cardiac abnormality and may occur as part of systemic or congenital disease. In developing countries, rheumatic heart disease is the most common cause, but bicuspid aortic valve and aortic root dilatation account for most of the cases in developed countries. Causes of aortic root dilatation include (1) Marfan syndrome and other related connective tissue diseases, and (2) aortitis secondary to syphilis, Behcet, Takayasu, Reiter syndrome, and ankylosing spondylitis. Endocarditis can lead to the rupture of leaflets, and vegetations on the valvular cusps can cause inadequate closure of leaflets. Aortic root dissection is a cause of acute AR.
The prevalence of at least mild AR is approximately 13% in men and 8.5% in women, and increases with age. Of asymptomatic people over the age of 55, 29% have AR of which a minority (13% of the patients with AR) have moderate to severe AR. In younger patients (23 to 35 years), the prevalence of AR is 1.3%.
AR can be classified as acute or chronic. Acute AR is secondary to acute aortic dissection, endocarditis, and deceleration injury (motor vehicle accident). With acute severe AR, the end-diastolic pressure in the left ventricle rises dramatically and the heart tries to compensate by increasing the heart rate and contractility (Starling law) to keep up with the preload. Often the compensatory mechanisms are insufficient to maintain the normal stroke volume and heart failure ensues. Chronic severe AR presents differently, as the patients remain asymptomatic for months to years while the heart compensates for gradual increased preload. The heart wall undergoes eccentric hypertrophy to decrease wall stress. Eventually, left ventricle systolic dysfunction supervenes and left ventricle end-diastolic pressure rises, resulting in symptomatic congestive heart failure. Timing aortic valve repair/replacement before irreversible myocardial dysfunction develops is of critical importance.
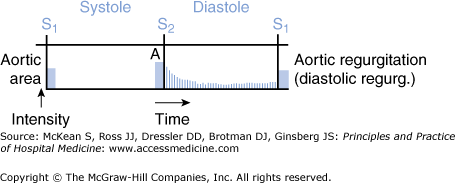
The signs and symptoms of AR depend on the acuity of disease. In acute AR, the patient can present with sudden onset of pulmonary edema and hypotension or cardiogenic shock. Patients may present with signs and symptoms of myocardial ischemia or aortic root dissection. The left ventricular size is normal on exam, and the diastolic murmur may be short and/or soft due to equalization of diastolic pressure between aorta and ventricle. An apical diastolic rumble may be present (Austin Flint murmur, see below) (Figure 131-4). Pulse pressure may not be increased due to reduced systolic pressure. The transthoracic echocardiogram confirms the presence, severity, and etiology of AR. Transesophageal echocardiogram is one of the best tests that can be performed if aortic root dissection is suspected in the setting of acute AR.
Figure 131-4
Diagram representation of the heart murmur of aortic regurgitation. The murmur is a decrescendo murmur in early diastole that can also continue into late diastole when listening at the apex (Austin Flint murmur). (Reproduced, with permission, from LeBlond RF, DeGowin RL, Brown DD. DeGowin’s Diagnostic Examination, 9th ed. New York: McGraw-Hill, 2009. Fig. 8-39.)
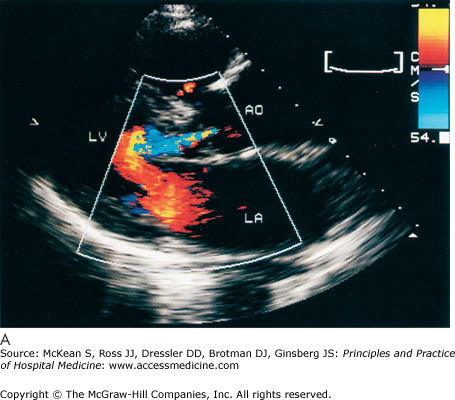
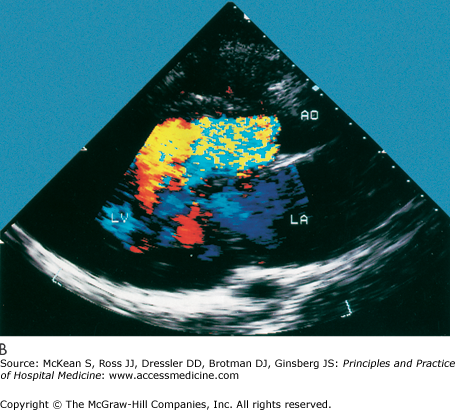
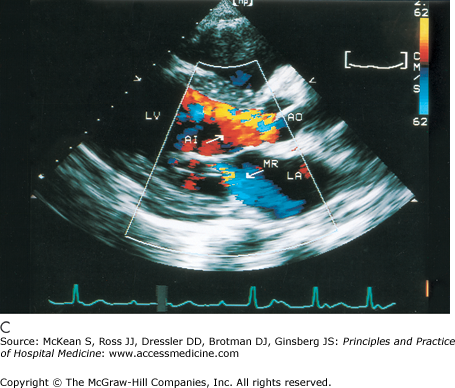
History and physical exam findings may be suggestive of AR (Table 131-2). Because physical signs are often not specific enough to judge the severity of the AR, transthoracic echocardiography is performed to better delineate disease severity (Figure 131-5). Echocardiography evaluates the valvular anatomy, aortic root, and impact of AR on ventricular size and function. Several echo indexes are used to assess severity. Color Doppler provides visualization of the origin of regurgitant jet and its width (color jet width > 65% of left ventricular outflow tract is severe AR). Continuous wave Doppler can estimate the degree of AR, assuming the left ventricular end diastolic pressure is not very elevated (pressure half time < 250 msec is severe AR). Diastolic reversal of flow in the descending aorta can also be seen by echo and is indicative of severe AR.
| Aortic Regurgitation Symptoms | Physical Exam Findings |
|---|---|
| Decreased exercise tolerance, shortness of breath on exertion, chest pain, syncope/near syncope, heart failure |
|
Figure 131-5
Cardiac echocardiography in the parasternal long axis view shows mild (A) and severe (B and C) aortic regurgitation by color doppler (seen as turbulent color flow originating from aortic valve). Ao, aorta; AI, aortic insufficiency; LV, left ventricle; LA, left atrium; MR, mitral regurgitation. (Reproduced, with permission, from Fuster V, O’Rourke RA, Walsh RA, et al. Hurst’s the Heart. 12th ed. New York: McGraw-Hill, 2008. Fig. 16-64.)
|
If the results of echocardiogram (transthoracic and transesophageal) are of insufficient quality or if the impact of the AR on the patient is unclear, other tests can be ordered. In chronic severe AR, treadmill exercise testing is helpful to assess the functional status and symptomatic response in sedentary patients. Multi-gated Acquisition Scan (MUGA) or magnetic resonance imaging (MRI) can be used to assess left ventricular function. Fluoroscopic angiography evaluates AR and concomitant coronary disease preoperatively, which is particularly useful in patients undergoing valvular surgery that have risk factors for coronary disease (men > 35 years, premenopausal women > 35 years, and postmenopausal women).
Acute severe AR is often a surgical emergency. Surgery should be performed as soon as possible in patients with hemodynamic instability or congestive heart failure, particularly if medical therapy is ineffective. Recommended initial management for patients with acute AR includes airway management (intubation), positive inotropic agents (dobutamine), and a vasodilator therapy (nitroprusside).
Chronic AR has a protracted course and patients may remain asymptomatic for decades. Many patients with mild or moderate AR remain stable and may never require corrective surgery. Treatment of chronic AR depends on five factors: (1) severity, (2) symptoms, (3) left ventricular function, (4) left ventricular dilatation, and (5) operability. Medical therapy with vasodilators (hydralazine, nifedipine, and angiotensin converting enzyme inhibitors) does not improve mortality. Aortic valve replacement is performed in the majority of patients requiring surgery, though aortic valve repair may be possible in selected patients at specialized centers. The majority of the patients show reversal of left ventricular dilatation and improvement in ejection fraction (EF). Preoperative left ventricular function is the best predictor of long-term prognosis in patients undergoing surgery. Patients with normal preoperative EF or a brief duration of left ventricular dysfunction (less than 14 months) have greater improvement in left ventricular dimensions and early and late postoperative improvement in left ventricular function. Patients with impaired preoperative ventricular function have a greater risk of developing congestive heart failure postoperatively. In general, surgery should be performed before the EF is ≤ 50% and/or before the left ventricular end systolic dimension is ≥ 55 mm to preserve the left ventricle even in asymptomatic patients.