Small intestine
Large intestine
Proximal
Distal
Competent ileocecal valve
Non-competent ileocecal valve
Anorexia
Present
Variable
Variable
Variable
Vomiting
Present: bilious, large quantity, non-malodorous
Usually present: feculent/particulate, small volume, foul smelling
Absent
May be present
Pain
Peri-umbilical, colicky pain, early symptom
Localized, deep visceral pain with pain-free intervals, late symptom
Localized, deep visceral pain with pain-free intervals, late symptom
Localized, deep visceral pain with pain-free intervals, late symptom
Distension
Usually absent
Present
Present
Present
Plain abdominal films will be consistent with bowel obstruction. Although CT is rarely necessary to diagnose bowel obstruction, it may be helpful for identifying malignancy as a potential cause. Moreover, information from CT assists in management decisions and is often helpful for locating the site of obstruction or determining if there is multilevel involvement or a single, discrete segment of bowel that may be amenable to obstruction or bypass. The presence of massive ascites, carcinomatosis, evidence of bowel compromise, or multilevel intestinal involvement will influence surgical decision making if seen on CT. Multidetector CT with thin sections and both oral and intravenous contrast enhancements is the imaging modality of choice for detecting carcinomatosis. There are limits to the sensitivity of imaging. For example, peritoneal implants <5 mm and those in certain anatomical locations, including the small intestine serosa, may not be detected on CT, so their absence in imaging does not exclude diagnosis of carcinomatosis [7]. MRI could be considered for some patients. Although MRI has comparable detection sensitivity with multidetector CT for identifying peritoneal deposits >1 cm, fat-suppressed, delayed gadolinium-enhanced MRI is more sensitive than CT for detecting subcentimeter deposits, including those <5 mm, and deposits on bowel serosa or other anatomically difficult sites [7].
Treatment Decisions and Patient Selection
Surgical decision making for MBO requires the highest degree of clinical judgment and thoughtful communication with patients and families. Perhaps the most fundamental decision is the one regarding the need and benefit of surgical intervention. An algorithm summarizing decision making is presented in Fig. 26.1. Because MBO rarely requires intervention within the first few hours of presentation, there is usually adequate time to counsel the patient and family. Surgical intervention for MBO aims to reduce symptoms and improve the quality of life but does not address the underlying incurable malignancy. However, relieving the obstruction may improve nutritional intake, prevent perforation and ischemia and prolong life. One retrospective, single institution study of a heterogeneous population of patients with MBO compared 324 patients who had surgery with 199 patients who did not. They found that surgical patients had lower rates of reobstruction (18 % vs. 35 %), longer mean time to reobstruction (223 days vs. 36.4 days), and longer duration of survival (331 days vs. 174 days) [2]. Other studies using population-based data for patients with ovarian and colorectal cancer found increased survival among patients managed surgically, but did not demonstrate a lower rate of reobstruction [5, 6]. Surgery may also act as a bridge to allow further disease-directed treatments, including additional chemotherapy [4]. However, because there are currently are no randomized controlled trials of MBO management, favorable outcomes may be subject to selection bias.
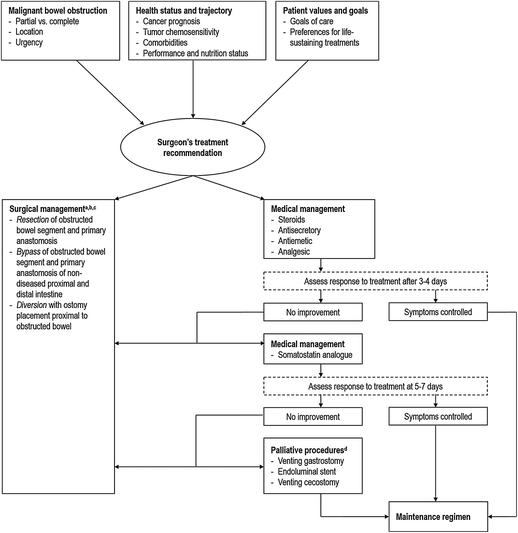

Fig. 26.1
Treatment decision-making algorithm for malignant bowel obstruction
Patient factors associated with worse surgical outcomes include advanced age, poor nutritional status, comorbidities, persistent ascites, poor performance status, prior abdominal radiation therapy, and failed prior surgery for MBO (see Table 26.2). Poor nutritional status and poor performance status are each associated with 3 times higher odds of dying after surgery [8]. Although not absolute contraindications to surgery, these factors greatly increase surgical risk, and potential benefits of surgery must be weighed against increased potential for complications. Patients who have diffuse peritoneal carcinomatosis and those with multilevel obstruction are unlikely to receive benefit from surgical intervention. Peritoneal carcinomatosis is associated with high rates of serious complications (7–44 %), mortality (6–32 %), reobstruction (6–47 %), and readmissions (38–74 %) with limited survival [3]. Therefore, surgery is not recommended for these patients.
Table 26.2
Poor prognostic factors for surgical treatment of bowel obstruction
Patient factors | – Advanced age – Chronic comorbidities – Poor performance status – Poor nutritional status (low albumin, progressive weight loss) – Prior abdominal radiation |
Disease factors | – Disease progression despite chemotherapy – Multilevel obstruction – Diffuse peritoneal carcinomatosis – Palpable masses – Persistent ascites – Complete obstruction (vs. partial obstruction) |
Given the overall poor prognosis for patients with incurable cancer and MBO, a thorough discussion should take place before surgery between the surgeon, patient, and family to elicit the patient’s goals for treatment and set reasonable expectations for recovery and outcomes. Patients have differing degrees of disease awareness, so it is helpful to initiate the conversation by determining the patient’s and family’s understanding of their disease and prognosis. This will allow the surgeon to place the acute MBO in the context of underlying disease. The surgeon should then inform the patient and family about the acute problem, explaining the disease course of MBO and its likely impact on the patient’s health trajectory. If at all possible, it is advisable to engage other treating clinicians, including the patient’s oncologist, in discussions about prognosis, potential outcomes, and treatment decisions [9].
The American College of Surgeons has built an online risk calculator based on NSQIP data to help surgeons and families understand the range of morbidity and mortality associated with certain known comorbidities, urgency of the operation, age, and specifics of the operation. It is worth running the risk calculator to see whether the family’s or surgeon’s expectations are founded on the data available to this risk calculator, which should evolved over time (http://www.riskcalculator.facs.org/).
The risks, benefits, and burdens associated with each treatment option should be described clearly. Discussing treatment burden is of particular importance because patients may experience prolonged recovery, substantial hospitalization, and discharge to institutional settings which, in the context of limited survival, may be incongruent with their priorities for end-of-life care. Determining the appropriateness of treatment options requires considering the ability of each treatment to achieve the individual patient’s goals for care. In addition to speaking about specific treatments for MBO, surgeons should inquire about the patient’s general priorities for health care, and goals for comfort, quality, and duration of life. This conversation should identify specific health states or life-sustaining treatments that would be unacceptable to the patient as well as activities and milestones that are most important. Existing advance directives and healthcare proxy designations should be reviewed.
The surgeon should then make a recommendation, synthesizing the patient’s goals with clinical data. Patients who consent to surgery must be counseled on the element of uncertainty prior to the operation. Although imaging and history may guide surgical planning, the definitive decision must be made intra-operatively, so their consent or rejection of each potential surgical approach should be determined. The care plan for patients managed non-surgically should include a plan to evaluate success at specific times and revisit treatment options. This conversation and the treatment plan should be documented in the medical record and shared with other clinicians and the patient’s surrogate decision makers.
Surgical Approaches
Surgical intervention in the face of MBO should be directed at achieving the lowest risk of complications with the highest chance of meeting patients’ goals for care (see Table 26.3). Decisions about future chemotherapy, including intraperitoneal regimens after cytoreductive operations, will be guided by the patient’s performance status, prior experience with chemotherapy, and tumor type. Thus, surgical decisions predicated on future chemotherapy should be made in consultation with the patient’s oncologist. For patients with MBO from a colorectal primary and unresectable metastatic disease, resection of the primary tumor is associated with longer survival compared with endoluminal stenting. Therefore, tumor resection with negative margins is the procedure of choice for patients with MBO due to a single lesion. If this is not possible, there is little benefit conferred from tumor debulking in regard to overall prognosis for patients with incurable disease.
Table 26.3




Surgical approaches to malignant bowel obstruction
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








