Ultrasound-Guided Intrathecal Pump Management
Hadi S. Moten
Brian Durkin
Introduction
Since the discovery of dorsal horn mu receptors, the goal of the spinal administration of opioids has been to minimize the amount of opioid needed while still adequately controlling a patient’s pain. In 1991, the U.S. Food and Drug Administration (FDA) approved the use of programmable pumps for drug infusion. Then, in 1995, the FDA approved the use of intrathecal morphine. This lead to a dramatic increase in the use of intrathecal pumps (ITPs) to control pain.1 Currently, ITPs are used not only to control pain, but also to aid those plagued by spasticity. With the increased use of ITPs, as well as an increased typical duration of use, pain physicians find themselves faced with the need to frequently access and refill ITPs. Accessing the pump’s reservoir can become difficult due to a patient’s difficult anatomy, complications from the underlying disease pathology, or pump malposition.2 Traditionally, fluoroscopy has been utilized to aid in pump refills, but recently, ultrasound-guided refills have become more common. Ultrasound-guided ITP comes in two distinct varieties: soundassisted, where ultrasound is used to locate and mark the appropriate access point,3 and real-time ultrasound-guided, where ultrasound is continuously used not only to identify the location, but also to monitor the administration of medication.4
Refill protocol including images
Once the intrathecal pump has been successfully implanted, telemetry is utilized to interrogate the pump and identify the type of medication, concentration, and dosage. A frequent follow-up is initially required to identify appropriate dosage, assess pain control, and evaluate the patient for signs of potential complications. The pump is interrogated to ascertain the expected volume of medication in the reservoir. This is compared to the actual amount remaining in the reservoir by engaging the reservoir with a Huber needle and withdrawing all remaining medication.
Contents of a typical ITP refill kit include
Sterile drapes
Pump template
Huber needle
Syringes
Micropore filters
Tubing
Stop cocks
(Differences may exist between manufacturers and pump models.)
Refill process
Setup: The manufacturer’s kit is opened and prepared in a sterile manner.
Skin Examination: Look for any gross abnormalities, rashes, or signs of underlying pathology.
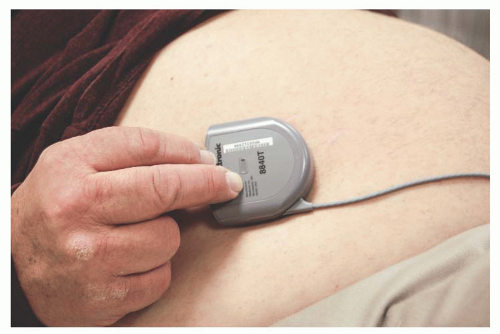
The pump is palpated, and its position is approximated (Fig. 73.1).
Computer Telemetry: The pump is interrogated, checked for malfunction, and the volume of medication expected to remain is noted.
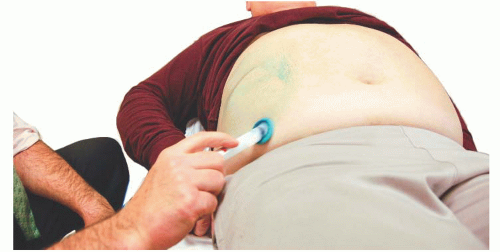
Preparation of Site: The area is prepared with pathogen appropriate solution. A wide prep is utilized to account for any area that may come in contact with the probe and allow for various approaches to the pump location. Often, the identified area is carefully prepped using sterile drapes (Fig. 73.2).
Probe Preparation: The ultrasound probe is prepped in a sterile manner, and a sterile lubricant is applied to the skin.
Pump Is Identified: The ultrasound probe is placed over the lubricated anticipated pump location. The pump reservoir, access port, and surrounding tissue are identified. The surrounding tissue is evaluated for signs of obvious pathology.
STandard Ultrasound Image: Basic landmarks when viewing a pump via ultrasound (Fig. 73.3).
Difficult Access: Demonstrates the difficulty in accessing the refill port in an ill patient that has a seroma over the refill port location.
Local Lidocaine Infiltration:




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree