CHAPTER 24 Trigger Point Injections
Description
Terminology and Subtypes
Trigger point injections (TPIs) refer to the injection of medication directly into trigger points. Trigger points are defined as firm, hyperirritable loci of muscle tissue located within a “taut band” in which external pressure can cause an involuntary local twitch response termed a “jump sign”, which in turn provokes referred pain to distant structures.1 Establishing a diagnosis of trigger points often includes a history of regional pain, with muscular overload from sustained contraction in one position or repetitive activity, presence of a taut band with exquisite spot tenderness, reproduction of the patient’s pain complaint, and a painful limit to muscle stretch.2–4 Despite being an integral component to the definition of trigger points, it has been reported that the twitch response cannot reliably be established.5
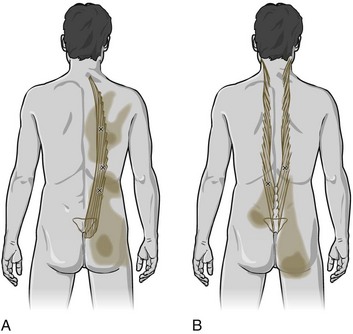
The two main types of trigger points are active and latent. Active trigger points can cause spontaneous pain or pain with movement, whereas latent trigger points cause pain only in response to direct compression.6 A pressure threshold meter, also termed an algometer or dolorimeter, is often used in clinical research to measure the amount of compression required to elicit a painful response in trigger points.7 Trigger points can be classified as central if they occur within a taut band, or attachment if they occur at a musculotendinous junction (Figure 24-1). When accompanied by other symptoms, trigger points may also constitute myofascial pain syndrome, one of the most frequent causes of musculoskeletal pain (Figure 24-2).8 Many often inaccurate terms have been used to denote trigger points, including Travell points, myofascial pain syndrome, myofascitis, fibrositis, myofibrositis, myalgia, muscular rheumatism, idiopathic myalgia, regional fibromyalgia, nonarthritic rheumatism, tendinomyopathy nonarticular rheumatism, local fibromyalgia, and regional soft-tissue pain.1,9
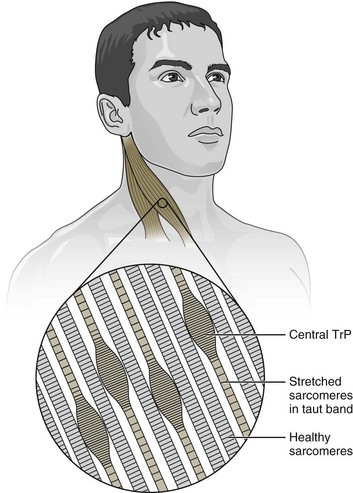
Figure 24-1 A central trigger point (TrP) located within a taut band of muscle.
(Modified from Muscolino JE: The muscle and bone palpation manual with trigger points, referral patterns, and stretching. St. Louis, Mosby, 2009.)
TPIs may be classified according to the substances injected, which may include local anesthetic, saline, sterile water, steroids, nonsteroidal anti-inflammatory drugs, botulinum toxin, 5-HT3 receptor antagonists, or even dry needling.10–38 Although this chapter focuses on TPIs for chronic low back pain (CLBP), trigger points may occur elsewhere in the body.
History and Frequency of Use
The German anatomist Froriep referred to tender spots occurring in muscles as muscle calluses in 1843; these points were called “myalgic spots” by Gutstein in 1938.39 Many other eponyms have been used to describe the same phenomenon. Studies have reported that 14.4% of the population of the United States has experienced myofascial pain, and suggested that 21% to 93% of all pain complaints were myofascial in origin.40,41 Although long thought to be separate entities, there was no clear delineation between myofascial pain syndrome and fibromyalgia until the American College of Rheumatology published diagnostic criteria for fibromyalgia in 1990.42 This milestone was not universally celebrated within the medical profession, and some have contended that both myofascial pain syndrome and fibromyalgia were the products of “junk medicine,” supported by poorly designed trials and unfounded theories, with the aim of legitimizing somewhat vague psychosomatic illnesses.39 Trigger points may also be present in fibromyalgia, osteoarthritis, rheumatoid arthritis, or connective tissue disorders.43
The term myofascial trigger point was coined and popularized by Janet Travell, who was the personal physician to President John F. Kennedy. Her contribution to medical pain management was primarily the study and description of myofascial pain with the publication, along with coauthor and physician David Simons, of the text Myofascial Pain and Dysfunction: The Trigger Point Manual in 1983.44 Travell and Simons continued to advance their proposed understanding of myofascial pain treatment and published a second edition of their manual in 1992.2 Although the method proposed by Travell and Simons for identifying and injecting trigger points became prominent, it was based largely on anecdotal observations and their personal clinical experience.39,45 The use of injection therapy for trigger points had previously been reported almost four decades earlier in 1955 by Sola and Kuitert, who noted that “Procaine and pontocaine have been most commonly used but Martin has reported success with injections of benzyl salicylate, camphor, and arachis oil.”46
Practitioner, Setting, and Availability
Any physician familiar with the localization of trigger points and the use of therapeutic musculoskeletal injections may perform TPIs. However, these injections are probably best performed by physicians with postgraduate education in musculoskeletal anatomy, and a greater understanding of orthopedic and neurologic disorders. Although a few states currently allow physical therapists or naturopaths to perform dry needling, most states do not permit such injections by nonphysicians.47 This intervention is typically performed in private outpatient clinics, but can also be offered in specialty pain management or spine clinics. TPIs are widely available throughout the United States.
Procedure
TPIs usually require that the patient wear a medical gown and lie prone on a treatment table. Patient positioning should be comfortable to minimize involuntary muscle contractions and facilitate access to the painful areas. Trigger points are first located by manual palpation with a variety of techniques (Figure 24-3). The highest inter- and intra-examiner reliability for locating trigger points was achieved with pressure threshold algometry.48,49 Once trigger points are located and marked with a skin pen, the skin is generally prepared with a standard antibacterial agent such as isopropyl alcohol or betadine solution. The needle size used for TPIs is typically quite small, frequently 25 or 27 gauge (G), but needles as large as 21G have been reported.10-12,14,18-20,24,26,32,50 The length of needle used is dependent on the depth of the trigger point through subcutaneous tissue, but is commonly from 0.75 inches to 2.5 inches.10,12,14,18,20,46,50–52 Acupuncture needles may be used for dry needling of trigger points, using 0.16 × 13 mm for facial muscles to 0.30 × 75 mm for larger or deeper muscles.
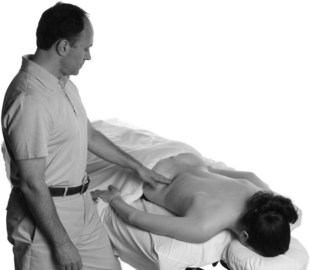
Figure 24-3 Palpation of trigger points prior to injections.
(From Muscolino JE: The muscle and bone palpation manual with trigger points, referral patterns, and stretching. St. Louis, Mosby, 2009.)
The number of trigger points injected at each session varies, as does the volume of solution injected at each trigger point and in total. A common practice is to use 0.5 to 2 mL per trigger point, which may depend on the pharmacologic dosing limits of the injected mixture.11,12,14,15,19–21,26,32,33,50 For example, the total dose of Botox A administered during TPIs ranged from 5 to 100 units/site, for 10-20 sites, up to a total of 250 units.18,22,24,25 Lidocaine is a frequently used local anesthetic for TPIs; a dilution to 0.2% to 0.25% with sterile water has been suggested as the least painful on injection.11,13-15,18,26 Other studies have used ropivacaine or bupivacaine 0.5% with or without dexamethasone.12
The injection technique recommended by Hong and Hsueh for trigger points was modified from that proposed by Travell and Simons.13,50 It described holding the syringe in the dominant hand while palpating the trigger point with the thumb or index finger of the opposite hand (Figure 24-4). Needle insertion was into the subcutaneous tissue adjacent to the trigger point at an angle of 50 to 70 degrees to the skin, aiming at the taut band. Multiple insertions in different directions from the subcutaneous layer were “fast in” and “fast out” to probe for latent trigger points. Each thrust coincided with the injection of 0.02 to 0.05 mL of injectate, up to a total of 0.5 to 1 mL in each trigger point. Compression of the point for 2 minutes allowed hemostasis, which was followed by stretching of the muscle. They noted that the best responses to injection were found when the “local twitch response” was provoked by impaling the active point.13
Theory
Mechanism of Action
The physiology of trigger points themselves is controversial, and therefore the mechanism of action through which injections aimed at trigger points may relieve pain is unknown.39 In 1979, a theory of “diffuse noxious inhibitory control” was suggested where noxious input from nociceptive afferent fibers inhibited dorsal horn efferents as a “counter irritant” from a distant location.53 Some support was given to this theory when subcutaneous sterile water improved myofascial pain scores after a brief period of severe burning pain at that site.54 Spontaneous electrical activity was found more frequently in rabbit and human trigger points.9,55 Simons56 theorized that the spontaneous electrical activity found in active trigger point loci was abnormal end-plate potentials from excessive acetylcholine leakage. This acetylcholine was thought to depolarize the postjunctional membrane, resulting in prolonged Ca++ release, continuous muscle fiber shortening, and increased metabolism.
Additionally, local circulation was thought to be compromised, thus reducing available oxygen and nutrient supply to the affected area, impairing the healing process. A muscle fiber “energy crisis” was hypothesized to produce taut bands. The concept of abnormal end-plate potentials was used to justify injection of botulinum toxin to block acetylcholine release in trigger points.57 McPartland has expanded on the idea of excessive acetylcholine by suggesting that congenital or acquired genetic defects in presynaptic, synaptic, or postsynaptic structures may contribute to an individual’s susceptibility to myofascial pain.45
Animal and human models suggest that the local twitch responses and referred pain associated with trigger points are related to spinal cord reflexes.34 Simons and Hong suggested that there are multiple trigger point loci in a region that consist of sensory (nociceptors) and motor (abnormal end-plates) components.63 By modifying the peripheral nociceptive response (desensitization), the nociceptive input to higher neurologic centers of pain and resulting increased muscle fiber contraction are blocked. The desensitization or antinociceptive effects by pressure, cold, heat, electricity, acupuncture, or chemical irritation relies on “gate-control theory” from Melzack.58,59 Local anesthetic also blocks nociceptors by reversible action on sodium channels.
Endogenous opioid release may play a role in TPIs. Fine and colleagues reported that the analgesic effects of TPIs could be reversed with intravenous naloxone.60 Mechanical disruption may play some role in breaking up trigger points.38,61 Spontaneous electrical activity, as originally observed, was later confirmed to be end-plate potentials.62 This finding was used to show that many traditional “ah-shi” acupuncture points corresponded to trigger points.63 Animal models also suggest the role of the autonomic nervous system related to phentolamine, an alpha-adrenergic agonist that inhibits sympathetic activation and decreases spontaneous electrical activity in rabbit myofascial trigger spots.64
Thermographic imaging evaluation has previously demonstrated elevated temperatures in the referral pain pattern of trigger points, suggesting increased local heat production from increased metabolism or neural activity.65 Gerwin and colleagues recently expanded on Simons’ integrated hypothesis for trigger point formation and proposed a complex molecular pathway whereby unconditioned muscle undergoes eccentric exercise or trauma, which results in muscle fiber injury and hypoperfusion from capillary constriction.66 Sympathetic nervous system activation further enhances this constriction and creates a hypoxic and acidic environment, facilitating the release of calcitonin gene-related peptide and acetylcholine. Additional proinflammatory mediators (e.g., adenosine triphosphate, serotonin, tumor necrosis factor-1a, interleukin 1, substance P, and Hþ ions) are then released from damaged muscle fibers, leading to activation of nociceptors and end-plate activity. Acetylcholine receptors are then up-regulated, resulting in more efficient binding, and producing taut bands. Neuroplastic changes in the dorsal horn may also activate neighboring neurons at lower thresholds, resulting in allodynia, hypersensitivity, and referred pain. The calcitonin gene-related peptide may be associated with this condition becoming chronic, as is hypothesized to occur in some patients with CLBP.
Indication
The indication for TPIs is CLBP with active trigger points in patients who also have myofascial pain syndrome that has failed to respond to analgesics and therapeutic exercise, or when a joint is deemed to be mechanically blocked due to trigger points and is unresponsive to other interventions.67 The best outcomes with TPIs are thought to occur in CLBP patients who demonstrate the local twitch response on palpation or dry needling.13,68 Patients with CLBP who also had fibromyalgia reported greater post-injection soreness and a slower response time than those with myofascial pain syndrome, but had similar clinical outcomes.50,69,70
Assessment
Before receiving TPIs, patients should first be assessed for LBP using an evidence-based and goal-oriented approach focused on the patient history and neurologic examination, as discussed in Chapter 3. Clinicians should also inquire about medication history to note prior hypersensitivity/allergy or adverse events (AEs) with drugs similar to those being considered, and evaluate contraindications for these types of drugs. Diagnostic imaging or other forms of advanced testing is generally not required before administering this intervention for CLBP.
Identification of trigger points is required before performing these injections and is generally performed with a thorough manual and orthopedic examination. However, insufficient training in trigger point examination likely impedes recognition of myofascial pain, and palpation generally has poor interrater reliability.2,44,71 Hsieh and colleagues reported difficulties when attempting to reproduce findings of taut bands and local twitch responses, both characteristics of trigger points, in the lower back.72 In a study of intra-rater reliability, local twitch response and referred pain varied from one session to the next while taut bands, tender points, and “jump sign” remained consistent.73 Likewise, Njoo and van der Does found that “jump sign” and “reproduction of pain” were much more reliable than “referred pain” in identifying myofascial pain.74 It is interesting to note that when Hong and colleagues compared referred pain response from needling and palpation, they found that only 53.9% of their patients had referred pain from palpation, compared with 87.6% when needling.35
Differentiating between the trigger points of myofascial pain syndrome and the tender points of fibromyalgia syndrome has also proven problematic. When clinicians were asked to examine patients with either myofascial pain, fibromyalgia, or healthy controls, the number of tender points identified was generally consistent.43 Even among experts in myofascial pain and fibromyalgia there was inconsistency in the number of taut bands, presence of referred pain, and local twitch responses reported. Asymptomatic subjects were reported to have as many latent trigger points as those with myofascial pain or fibromyalgia. This study prompted some clinicians to abandon the local twitch response to more reliably quantify tenderness with pressure thresholds, as reflected in the most current diagnostic criteria for trigger points.2–4
Pressure threshold is the minimum pressure that reproduces pain (or tenderness) in a suspected trigger point, and has been claimed to be an objective, reproducible, and reliable method for their detection.48,50,75–77 Fischer attempted to establish standard, normal pressure thresholds, which were found to be different for each gender and each muscle.76
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree