Tricuspid and Pulmonic Valve Disease
Dara K. Lee
Carlos A. Roldan
The physical examination remains the primary screening method for detection of tricuspid or pulmonic valve disease.
However, the accuracy of the physical examination for detecting and determining the etiology and severity of right-sided valve disease is low.
The limitations of the physical examination in the assessment of right-sided valve disease stem largely from the fact that the right-sided heart is a low-pressure system with low transvalvular gradients and, therefore, low audibility of tricuspid and pulmonic murmurs.
The murmur of tricuspid regurgitation (TR) can be mistaken for eccentric mitral regurgitation, and pulmonary regurgitation (PR) or pulmonary stenosis (PS) can be mistaken for aortic regurgitation or aortic stenosis, respectively.
As with left-sided lesions, acute right-sided regurgitant lesions may be clinically silent.
Finally, the physical examination cannot distinguish if valve regurgitation is due to a structurally abnormal valve (primary valve disease) or secondary to pulmonary hypertension or right ventricle (RV) or annular dilatation (secondary or functional valve disease).
Common Etiologies
Primary Right-Sided Valve Disease
Acquired Lesions
Rheumatic heart disease affects the right-sided valves in 30% to 50% of cases.
Worldwide, TR and/or tricuspid stenosis (TS) are predominantly caused by rheumatic heart disease (>90% of all cases). However, only 3% to 5% of cases of rheumatic heart disease have significant TR or TS.
Infective endocarditis due to intravenous drug abuse and infection of pacemakers or defibrillators are the most common causes of primary right heart valve disease in the United States.
Also, direct trauma to the tricuspid valve during placement of pacemakers or defibrillators or during endomyocardial biopsies is not an uncommon cause of primary TR.
Carcinoid tumors can cause TR and/or TS, though this is rare. These tumors may metastasize to the liver, resulting in elevated serotonin levels, which then cause thickening and retraction of the valve leaflets, resulting in mixed TR/TS.
Rarely, ergotamine alkaloids and diet pills containing fenfluramine-phentermine mimic the effects of carcinoid on the right heart valves.
Rarely, blunt chest trauma can cause primary TR or PR.
Acquired pulmonic valve disease is uncommon and is the valve least affected by rheumatic or carcinoid disease, or infective endocarditis.
Congenital Lesions
Congenital lesions include myxomatous valve degeneration or floppy valve syndrome. Thirty to 40% of patients with mitral valve prolapse have tricuspid valve prolapse as well.
Ebstein’s anomaly is characterized by apical displacement and dysplasia of the septal and posterior tricuspid leaflets; associated severe TR is common. This condition is also associated with patent foramen ovale, atrial septal defect, ventricular septal defect (VSD), PS, and/or accessory pathways.
Congenital pulmonic valve stenosis may be due to a unicuspid, bicuspid, or, rarely, a quadricuspid valve.
Most cases of congenital PS are valvular (approximately 90%) and rarely are subvalvular or supravalvular.
Congenital PS is usually an isolated anomaly but may be associated with tetralogy of Fallot, a VSD with
hypertrophic sub-pulmonic stenosis, or an atrioventricular canal.
Congenital PS can also be associated with peripheral pulmonary artery (PA) stenosis in Noonan or Williams syndrome.
Functional Right-Sided Valve Regurgitation
Mild TR and PR can be seen in up to 75% of healthy individuals.
Functional TR and PR are most commonly caused by pulmonary hypertension. Pulmonary hypertension leads to: high RV systolic pressure; RV and tricuspid annular dilatation with consequent tenting of tricuspid leaflets; right ventricular outflow tract (RVOT), pulmonic valve annular, and main pulmonary artery (PA) dilatation; and, consequently, to variable degrees of TR or PR, or both.
RV volume overload (due to left-to-right intracardiac shunt or primary TR or PR) may result in RV dilatation and superimposed functional TR or PR.
Table 10.1 Class I or appropriate (Score 7–9) indications for transthoracic and transesophageal echocardiography in tricuspid and pulmonic valve disease
Initial evaluation of murmur (diastolic murmurs, holosystolic murmurs, late systolic murmurs, murmurs associated with ejection clicks) in asymptomatic patients for whom there is a reasonable suspicion of valvular heart disease
Symptoms of dyspnea, shortness of breath, light-headedness, syncope, infective endocarditis, or other clinical evidence of structural heart disease
Abnormal chest x-ray, ECG, or elevation of serum BNP concerning for heart disease
Assessment of known or suspected adult congenital valvular heart disease, either in an unoperated patient or following repair/operation
Patients who have sustained or nonsustained SVT
Evaluation of hypotension or hemodynamic instability of suspected cardiac etiology
Initial evaluation of known or suspected native valvular regurgitation or stenosis
Routine (yearly) re-evaluation of an asymptomatic patient with severe native valvular regurgitation or stenosis with no change in clinical status
Re-evaluation of native valvular regurgitation or stenosis in patients with a change in clinical status
Initial evaluation of prosthetic valve for establishment of baseline after placement
Initial evaluation of suspected infective endocarditis (native and/or prosthetic valve) with positive blood cultures or a new murmur
Re-evaluation of infective endocarditis in patients with a virulent organism, severe hemodynamic lesion, persistent bacteremia, a change in clinical status, or symptomatic deterioration
Evaluation of a cardiac mass (suspected vegetation or tumor)
Initial evaluation of known or suspected right heart failure (systolic or diastolic)
Re-evaluation of known right heart failure (systolic or diastolic) to guide therapy in a patient with a change in clinical status
TEE to diagnose/manage endocarditis with a moderate or high pretest probability (e.g., bacteremia, especially staph bacteremia or fungemia)
TEE if persistent fever in a patient with an intracardiac device
ECG, electrocardiogram; BNP, B-type natriuretic peptide; SVT, supraventricular tachycardia; TEE, transesophageal echocardiography.
(Adapted from Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/HFSA/HRS/ASNC/SCAI/SCCM/SCCT/SCMR 2011 Appropriate use criteria for echocardiography. J Am Coll Cardiol. 2011;57:1126–1166.)
RV ischemia or infarction and RV arrhythmogenic cardiomyopathy can cause TR or PR.
Echocardiography
Indications for Echocardiography
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines and the American Society of Echocardiography appropriateness criteria recommend echocardiography (echo) in patients with (i) signs or symptoms that can be attributed to right-sided valve disease, (ii) conditions associated with right-sided valve disease, and (iii) follow-up of patients with known right-sided valve (1,2,3) (Table 10.1).
Echo determines the presence, mechanism, and severity of right-sided valve disease and consequent RV enlargement, hypertrophy, and systolic or diastolic dysfunction, which may also exacerbate tricuspid or pulmonic valve disease.
Transesophageal echocardiography (TEE) is in general of less important diagnostic value in right-sided valve disease than in left-sided valve disease.
Tricuspid Valve Regurgitation
Two-dimensional Echocardiography: Valve Morphology
Best Imaging Planes
Transthoracic echo (TTE) parasternal long-axis view of the RV, parasternal basal short-axis view, and apical and subcostal four-chamber views.
TEE transgastric short- and long-axis views of the RV, midesophageal four-chamber view, and basal short-axis view longitudinal to the RVOT.
Key Diagnostic Features
Mild to moderate TR in a valve with thin leaflets and normal-appearing supporting structures suggests functional regurgitation.
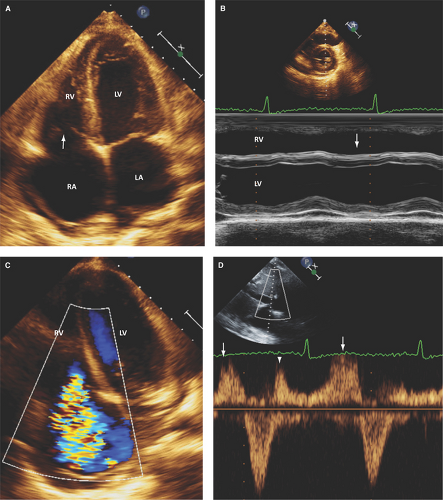
In rheumatic disease or other inflammatory valvulitis, thickening of leaflet tips, leaflet retraction, leaflet tethering from chordal thickening, fusion and retraction, and commissural fusion can cause both regurgitation and stenosis (Fig. 10.1).
In endocarditis, valve vegetations or perforations, or both, are often seen. Imaging of the tricuspid valve by TEE may be useful in infective endocarditis when small vegetations or valve perforations are suspected.
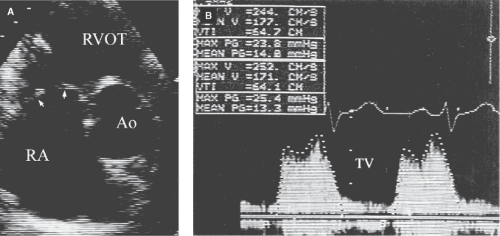
In carcinoid heart disease, the leaflets are thickened and retracted with a fixed orifice usually leading to predominant regurgitation and less severe stenosis (Fig. 10.2).
Approximately 30% of patients with mitral valve prolapse have prolapse of the tricuspid valve, leading to TR.
In Ebstein’s anomaly, >1 cm apical displacement and dysplasia of the tricuspid septal and posterior leaflets are seen.
In tricuspid valve dysplasia, significant thickening or calcification of the annulus or leaflets may be seen.
Right heart chamber enlargement and abnormal septal motion due to RV volume overload are seen with moderate or severe TR (Figs. 10.1A,B, 5.1B and 5.2D).
Three-dimensional Echocardiography: Valve Morphology
Best Imaging Planes
Those routinely obtained with two-dimensional (2D) TTE and TEE for online or offline reconstruction of RA and RV en face views of the tricuspid valve.
Key Diagnostic Features
Real-time three-dimensional (RT3D) TTE and TEE may provide a detailed structural assessment of the tricuspid annulus shape, diameters, and area; tricuspid valve leaflet shape and mobility; and tricuspid valve commissures (4,5).
Also, RT3D echo allows quantification of the degree of annular dilatation and leaflet tenting in functional TR associated with pulmonary hypertension (6).
In rheumatic tricuspid valve disease, RT3D echo can assess the extent of individual tricuspid valve leaflet pathology and assess the extent of commissural fusion (7).
RT3D echo may better characterize the structural abnormalities associated with Ebstein’s anomaly (8,9) and carcinoid heart disease (10,11).
Also, RT3D echo allows determination of the mechanisms of pacemaker- or defibrillator-related TR as (i) lead impingement of the tricuspid valve leaflets (39%), (ii) adhesion of the device leads to a tricuspid valve leaflet (34%), (iii) leaflet perforation (17%), and (iv) entanglement of the tricuspid valve apparatus (10%) (12,13).
In addition, RT3D echo allows better assessment of tricuspid valve infective and noninfective masses (14).
Doppler Echocardiography for Assessment of the Severity of Tricuspid Regurgitation
Pulsed and Continuous Wave Doppler: Key Diagnostic Features
Pulsed Doppler is highly sensitive for detecting TR, but not for defining its severity.
In moderate TR, there is reduction in the pulsed wave Doppler systolic flow of the hepatic veins; in severe TR, absent or reversed systolic flow is seen (Fig. 10.1D and Table 10.2).
In severe TR, the continuous wave Doppler envelope is as dense as the tricuspid inflow signal. Also, a V wave (reduction in velocity or concave configuration during the latter half of systole of the Doppler envelope) is seen as RV and right atrium (RA) pressures equalize (15).
In severe TR, a tricuspid inflow velocity ≥1.0 m/second is common.
Color Doppler: Diagnostic Methods and Key Diagnostic Features
TR jet area and proximal flow convergence zone on echo, as compared to RV angiography, differentiate mild to moderate (grade 1 to 2) TR from severe TR (grade 3 or 4).
The proximal isovelocity surface area (PISA) radius at an aliasing velocity of 28 or 41 m/second is of comparable diagnostic value to jet area or jet length, but all are better than the ratio of jet area to RA area.
Jet length >5 cm
Jet area >10 cm2
Jet area to RA area ratio >40%
PISA radius >10 mm with a Nyquist limit of 30 cm/second
PISA radius ≥7 mm with a Nyquist limit of 40 cm/second
Vena contracta width ≥7 mm
Pitfalls
The size and length of a regurgitant jet are related to momentum, which is the product of mass and velocity. Velocity increases relative to the pressure gradient across a valve. Because the RV is a low-pressure chamber, regurgitation of a similar volume of blood appears smaller in the RA than in the left atrium (LA).
In severe pulmonary hypertension, a regurgitant jet may appear larger and longer in relation to the RA despite a smaller regurgitant volume.
Because of the potential pitfalls in assessing the severity of TR by Doppler imaging alone, it is important to complement it with morphologic and functional characteristics of the RV and RA (Table 10.2).
Tricuspid Valve Stenosis
Two-dimensional Echocardiography: Valve Morphology
Best Imaging Planes
TTE parasternal long-axis view of the RV, parasternal basal short-axis views, and apical and subcostal four-chamber views.
TEE transgastric short- and long-axis views of the RV, midesophageal four-chamber view, and basal short-axis view longitudinal to the RVOT.
Key Diagnostic Features
In rheumatic TS, leaflets tip and chordal thickening, retraction, and tethering occur.
In carcinoid syndrome, which causes mixed stenosis and predominant regurgitation, the leaflets appear diffusely thickened, retracted, and fixed (Fig. 10.2A).
Ergotamine alkaloids and diet pills containing fenfluramine-phentermine may mimic the effects of carcinoid on the right heart valves (17).
In Loeffler disease, diffuse thickening of the leaflets also results in stenosis and regurgitation.
Table 10.2 Stratification of tricuspid and pulmonic valve disease
Mild
Moderate
Severe
Parameter
Tricuspid Valve Regurgitation
Valve morphology
Normal
Normal/abnormal
Abnormal
RV, RA, IVC size
Normal
Normal or dilated
Dilated (annulus ≥4 cm) unless acute TR
CW jet
Less intense than inflow
Almost as dense as inflow
As dense as inflow with late delay
Jet area
<5 cm2*
5–10 cm2
>10 cm2
Jet/RA area
<20%
20%-40%
>40%
Vena contracta width
Small*
Probably <7 mm
>7 mm
Proximal isovelocity surface area radius
≤5 mm
6–9 mm
≥10 mm with a Nyquist limit of 30 cm/s; ≥7 mm with a Nyquist limit of 40 cm/s
Hepatic vein flow
Systolic dominance
Systolic blunting
Systolic reversal
Tricuspid Valve Stenosis
Mean gradient
<2 mm Hg
2–4 mm Hg
≥5 mm Hg 
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access