Fig. 21.1
(a) AP compression fracture, (b) left lateral compression fracture, and (c) AP compression fracture with vertical shear extension.
Table 21.1
Young and Burgess classification of pelvic fractures.
AP compression | |
Type I | Separation of pubic symphysis <2.5 cm and no posterior instability |
Type II | Separation of pubic symphysis >2.5 cm and posterior instability of anterior sacroiliac complex |
Type III | Complete disruption of sacroiliac joint involving both anterior and posterior complexes |
Lateral compression | |
Type I | Posterior-lateral directed force with oblique pubic rami fx. No pelvic instability |
Type II | Anterior-lateral directed force with pivot point on anterior SI joint causing pubic rami fx, internal rotation of anterior hemipelvis, external rotation of posterior hemipelvis, and rupture of posterior SI ligaments |
Type III | Severe ipsilateral internal hemipelvis rotation causing contralateral external rotation resulting in AP compression of contralateral side. Disruption of ipsilateral posterior SI ligaments and contralateral anterior SI, sacrotuberous, and sacrospinous ligaments |
Vertical shear | |
Vertical disruptive force on one or both sides of the pelvis lateral to midline. Associated with ligamentous disruption and pelvic instability | |
Complex | |
At least two different vectors of force causing injury pattern | |
Diagnosis and Management
Motor vehicle collision accounts for the majority of pelvic fractures [6–8]. In addition, motorcycle crashes, fall from a height, pedestrians struck, and crush injuries can all cause fractures to the bony pelvis [9, 10]. History and physical findings are often relatively nonspecific. Complaints range from pelvic pain to lower abdominal pain, hip pain, and back and thigh pain. Visual inspection of the pelvis may reveal soft tissue injuries, including abrasions, lacerations, contusions, or hematomas. An important part of the initial physical exam is to assess for pelvic stability. This should be done by exerting gentle manual pressure inward on the anterior superior iliac spines (Fig. 21.2). Any motion should be interpreted as pelvic instability. Rocking the pelvis is unwise, as if the pelvis is unstable, it will cause the patient significant pain and almost certainly result in additional blood loss. An inspection of the perineum is also important. If a urethral injury is suspected based on the presence of blood at the meatus, a retrograde urethrogram can be performed (Fig. 21.3). Vaginal and rectal exams are likewise important to rule out the possibility of an open pelvic fracture.
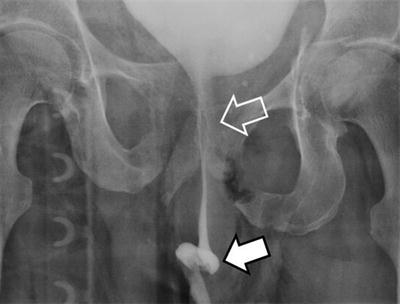

Fig. 21.2
Direction of gentle manual compression on the anterior superior iliac spines to assess for pelvic instability.

Fig. 21.3
Retrograde urethrogram demonstrating contrast extravasation from an irregular discontinuous anterior urethra (solid arrow) and stretch injury of the membranous and prostatic urethra (open arrow).
An AP film of the pelvis should be obtained in all patients with polytrauma, regardless of hemodynamic status. While this is helpful for predicting associated injuries and predicting outcome in patients awake and alert without concerning mechanism, those without complaints or physical findings may not need a pelvic film [11–14]. Additional imaging is almost always obtained with CT scanning, though in selected cases, inlet and outlet views of the pelvis as well as Judet views may be helpful [15–19].
Management of Pelvic Fracture Hemorrhage
Patients who present with pelvic fractures and are hemodynamically unstable can present the greatest challenge. Hemorrhage may be retroperitoneal in nature from the pelvic fracture, from other sites, or from both. Rapid assessment is important to identify all body cavities in which the patient may be losing blood. While covered elsewhere in more detail, a FAST or an eFAST may be very helpful in identifying intra-abdominal or intrathoracic hemorrhage, it will also help identify pneumothorax and/or hemopericardium, both of which can contribute to hemodynamic instability. A chest X-ray may also be helpful. Hemorrhage into muscle compartment is usually identified on physical examination and external hemorrhage is identified by history.
Patients who have a pelvic fracture and significant hemorrhage, either into the thorax or abdomen, pose a special problem. While patients with pelvic fractures are certainly at risk for retroperitoneal hemorrhage, not all of them are bleeding into their retroperitoneum. In general, patients with intra-abdominal hemorrhage, particularly if large volume on FAST, are best served with diagnostic abdominal exploration. At the time of laparotomy, the size of the pelvic hematoma can be ascertained by direct inspection.
Fracture pattern may be helpful in identifying patients more likely to be bleeding from their abdomen. A large retrospective study found that 85 % of hypotensive patients with hemoperitoneum and a stable pelvic fracture categorized as a lateral compression (LC1 or APCI) had intra-abdominal bleeding as the source of their hypotension. Conversely, 59 % of the patients with hemoperitoneum and an unstable pelvic fracture (LC2, LC3, APC2, APC3, or vertical shear) had large pelvic volume bleeding. The authors concluded that laparotomy should be performed first in the hypotensive patient with a stable fracture but consideration given to angiography before laparotomy in the patient with hemoperitoneum in an unstable pelvic fracture [20].
External compression is an important technique to control pelvic fracture hemorrhage. This can be accomplished in many ways. In a resource-poor environment, simply placing a sheet around the patient can help. It is ideal if the bedsheet is placed on the stretcher, the patient then placed on top of the bedsheet, and the sheet then crisscrossed across the patient’s pelvis. This should be tied down as tightly as possible to reduce the bony elements (Fig. 21.4).


Fig. 21.4
Application of a bedsheet as an external binder with the tails of the sheet crisscrossed across the pelvis.
In the past, emergency external fixation was often used to achieve pelvic bony stability (Fig. 21.5). While this was placed in the emergency department in some institutions, more commonly, it was placed in the operating room. Thus, its use as an emergency therapy was, in some ways, negated. In certain institutions, this remains a possibility. It should be recognized that in patients with grossly unstable pelvic fractures, external fixation may reduce the anterior elements but, in fact, widen the posterior elements, causing increased hemorrhage.
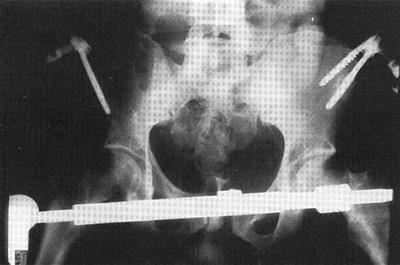

Fig. 21.5
Radiographic image of pelvic external fixator with pelvic reduction and stabilization.
In Europe, placement of a C-clamp is common (Fig. 21.6). This is not used very often in the United States. In those institutions, the C-clamp is placed blindly in the emergency department. In skilled hands, this is almost certainly safe. However, in those inexperienced, the potential visceral injury from poor placement of the pin must be recognized. Again, if this is going to be placed in the operating room under fluoroscopic control, its use as an emergency hemostatic maneuver is negated.
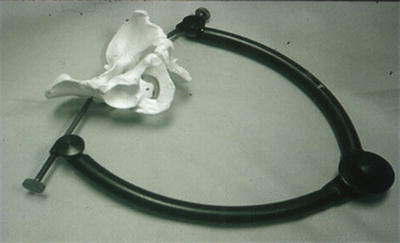

Fig. 21.6
Image of a C-clamp. Note the pins required for securing the device.
In the United States, virtually all of these hemostatic techniques have been replaced with the use of the pelvic binder [21–23]. The pelvic binder is a Velcro-based device which is placed around the patient’s pelvis. It is important to place the device low enough over the greater trochanters of the femurs (Fig. 21.7). The binder should not be placed over the abdomen. The binder can then be laced up and even pressure placed to reduce the bony elements of the fractured pelvis. Unlike the C-clamp or external fixator, the binder is easily placed in the resuscitation bay, often, while other resuscitative maneuvers are ongoing. In addition, this reduces all portions of the pelvis equally; posterior elements cannot be displaced. The pressure exerted by the binder can be increased or reduced depending on the clinicians’ wish. Physicians have to be cognizant of the length of time that the binders have been in place, as these devices have the potential of pressure ulceration, so the skin should be routinely inspected if applied for any length of time [24].
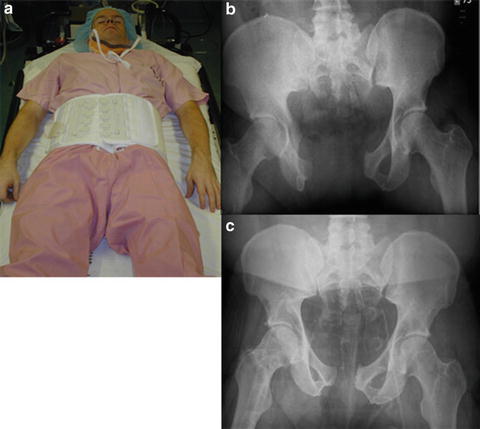

Fig. 21.7
(a) Pelvic binder application. Note the improperly placed binder over the abdomen instead of the greater trochanters. “Open book” AP compression fracture (b), with fracture reduction following proper placement of pelvic binder over the greater trochanters (c).
Regardless of the method used, the most important initial hemostatic maneuver in a fractured pelvis is to reduce the bony elements. This closes down the pelvic volume and almost certainly reduces pelvic venous bleeding. In addition, if fracture fragments are reduced, bony bleeding is almost certainly reduced. External compression likely has little effect on arterial hemorrhage.
There is little downside to blanket application of the binder. Certainly in patients with badly displaced AP compression fractures (open book fractures), application of the binder can be highly efficacious and even lifesaving. The same is not true in patients with lower-grade lateral compression or vertical shear fracture pattern. However, even in those people, there is some rationale for placing the binder. Patients with pelvic fractures commonly require transport to the hospital. Keeping the fracture fragments reduced with the binder may reduce additional bleeding when patients are transferred to and from the stretcher for various diagnostic tests and/or therapeutic maneuvers.
Angiographic Embolization
For over 40 years, angiography has been used to treat pelvic fracture hemorrhage. Angiography is diagnostic and embolization therapeutic (Fig. 21.8). Patients with evidence of significant pelvic hemorrhage, particularly those who are hypotensive following pelvic stabilization or have no other identifiable source for their hemodynamic instability, should undergo immediate angiography [25, 26]. Transcatheter embolization in the unstable patient has a reported success rate of 95 % in obtaining hemorrhage control [27]. Consideration should be given for repeat angiography and persistent pelvic bleeding in patients who have continued transfusion requirements or unexplained hypotension [28].
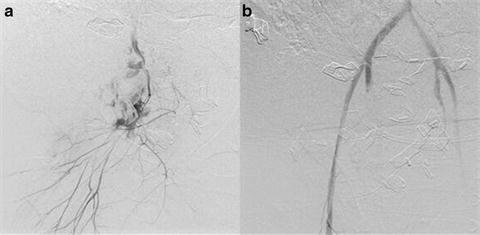

Fig. 21.8
(a) Contrast extravasation for right internal iliac artery and (b) post-angiographic embolization with resolution of extravasation.
In the hemodynamically stable patient, pelvic angiography and embolization may still have a role. Some recommend that all patients with pelvic fractures with contrast extravagation identified on CT scan undergo angiography to prevent hemorrhage [25, 29]. Other clinicians advocate a more selective approach to minimize nontherapeutic angiography in these stable patients [18, 30–32]. It would seem one clear indication for angiography should be a large pelvic hematoma (>500 cc’s) because of the associated risk for pelvic bleeding [33].
It is important that the surgeon caring for the patient have full understanding of the angiographic capability and availability. If access is limited, timely transfer of a stable patient to a center with angiographic capabilities is prudent, as delays are associated with increased mortality [34]. In the unstable patient, if angiography is unavailable, other means of hemostasis must be pursued.
In our institution, angiography is the primary means of obtaining hemostasis in patients with pelvic fracture bleeding, including those that are hemodynamically unstable. We have rapid access to angiography 24 h a day, 7 days a week. In addition, all hemodynamically stable patients with a large hematoma or large volume contrast extravasation undergo diagnostic angiography. Patients with evidence of concomitant solid organ (liver and/or spleen) bleeding can undergo visceral angiography at the time of pelvic angiography and have hemostasis in all vascular beds obtained.
Preperitoneal Packing and New Techniques
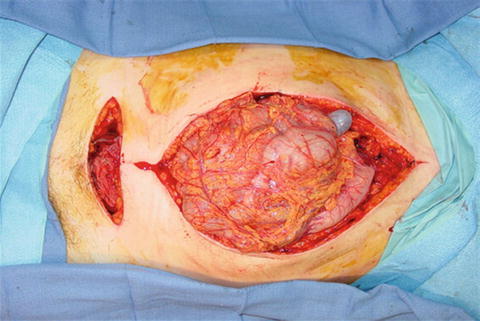
Unfortunately angiography may not be readily available, and in some hospitals nonexistent, so waiting for IR in the compromised patient is not an option. Preperitoneal packing as temporary, or potentially definitive, hemostasis can be lifesaving in this instance. This technique was originally described in Europe almost 20 years ago [35]. Recently this approach was modified to directly packing the anterior preperitoneal space [36]. To gain entrance to the space of Retzius, either a lower midline incision or a Pfannenstiel incision may be used. After the fascia is incised, the retroperitoneal space is bluntly dissected out laterally and posteriorly as far as possible (Fig. 21.9). While performing this maneuver, it is important not to breach the peritoneum and release its tamponade effects. This dissection is often created by the hematoma making it fairly easy to gain access to the retroperitoneum. Large clot is then evacuated and laparotomy pads are placed laterally to compress the iliac vessels. It is classically taught to place three laparotomy pads on either side; however, we place as many as needed to achieve tamponade.
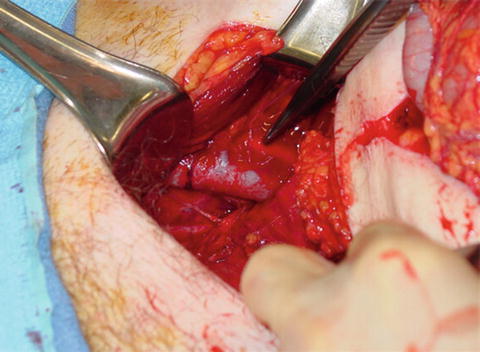

Fig. 21.9
Intraoperative exposure of the retroperitoneum. Note the identification of the iliac vessels.
Several studies have shown that preperitoneal packing directly resulted in a reduction in blood transfusion requirements and a reduction in the need for angioembolization [37, 38]. Preperitoneal packing can be combined with laparotomy in those unstable patients with both intra-abdominal and retroperitoneal hemorrhage. For a combined approach, the midline laparotomy incision is stopped midway between the umbilicus and pubic symphysis. Preperitoneal packing is then done through a separate Pfannenstiel incision (Fig. 21.10).