3 Treating neuropathic pain
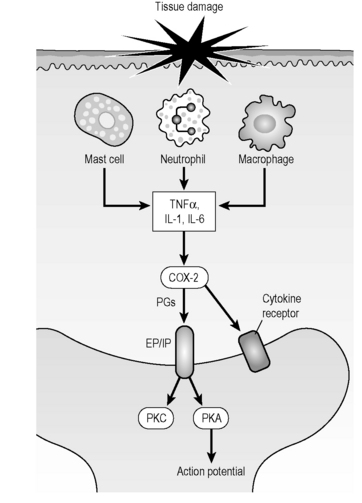
Unlike acute pain from trauma which is mediated by the firing of primary afferent nociceptors, chronic neuropathic pain is mediated by inflammation in the nerves through the action of the inflammatory cytokines IL-1, IL-6, TNF-α, and substance P (Meyers 2006, Zieglgansberger 2005, Tal 1999).
FSM has been shown to reduce inflammatory cytokines while treating the pain of fibromyalgia associated with cervical spine trauma which is thought to be neuropathic. One specific frequency combination has been shown to reduce IL-1 (330 to 80pg/ml, p = 0.004), Il-6 (239 to 76pg/ml, p = 0.0008), TNF-α (305 to 78,p=0.002), and substance P (180 to 54pg/ml p = 0.0001) and to increase endorphins (8.2 to 71.1pg/ml, p = 0.003) Pain scores were reduced from an average of 7.3±1.2 to 1.3±1.1 in 45 of 54 patients (p = 0.0001) (McMakin 2005). The treatment that reduced the pain also reduced cytokines.
Studies have shown an association between induction of Cox-2 increased prostaglandin release and enhanced nociception in neuropathic pain. Expression of Cox-1 and Cox-2 in primary afferents and in the spinal cord suggests that NSAIDs act there by inhibiting synthesis of prostaglandins (Zieglgansberger 2005, Tal 1999, Bennett 2000).
A controlled trial using a mouse model for lipoxygenase (LOX) mediated inflammation demonstrated 62% reduction in ear swelling in mice treated with frequency-specific microcurrent (FSM) using 40Hz and 116Hz when compared with the controls. COX mediated inflammation was reduced in the same mouse model by 30% which was equivalent to the prescription anti-inflammatory injectable Toridol when it was tested using the same mouse model. FSM experimentation demonstrated the result to be reproducible, application time dependent, and specific as other FSM frequencies had no effect on the model (Reilly 2004).
When the nerve becomes inflamed calcium ions flow into the nerve through voltage gated ion channels (Winquist 2005). The open channels create impulses interpreted as pain, the pain impulses travel up the nerve to the spinal cord and up the spinal cord to the pain processing centers in the brain.
Any inflamed tissue eventually experiences calcium influx and then fibrosis. Inflamed neural tissues are no exception. The concept of mobilizing fibrosed neural tissue and the surrounding fascia or dura has been well explored by Butler (1989a,b, 1991). FSM has well documented success in modifying scar tissue (Huckfeldt 2003) and its use in neural mobilization provides dramatic improvements over manual mobilization techniques both in comfort and in speed of response. The only frequency combinations observed to have an effect on neurofibrosis are those for scar tissue, sclerosis and fibrosis combined with the frequency for the nerve as a target tissue. The frequency to reduce pain does not reduce fibrosis or increase range of motion. If the nerve is traumatized by too vigorous stretching only the frequency to reduce inflammation will reduce the resulting pain.
Types of nerve pain
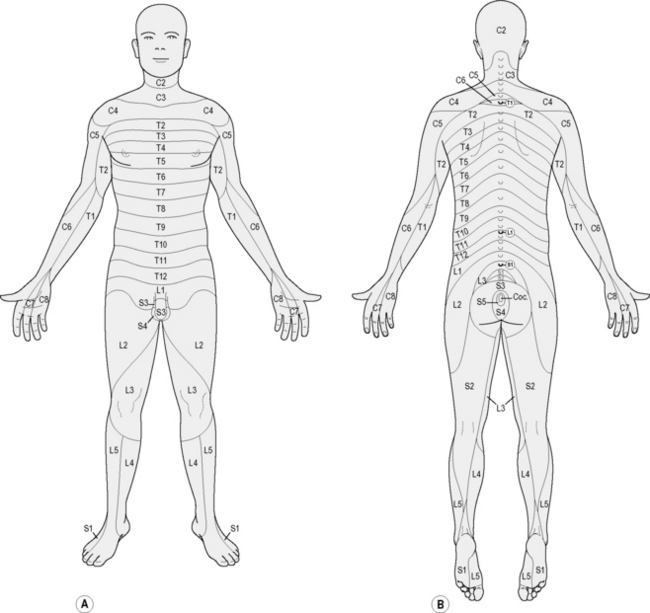
Nerves come out of the spinal cord as nerve roots and describe sensory patterns known as dermatomes; cutaneous nerves are branches of dermatomal nerves and provide sensation to specific areas of the skin. Nerves become pain generators when they are injured. A complete description of all types of neuropathic pain and their mechanisms is beyond the scope of this text and the reader is referred to Neurology in Clinical Practice (Bradley 2000) for a comprehensive treatment of the subject.
Basic physical examination to evaluate nerve function
Reflex testing
A normal reflex is graded as +2/4 and is characterized by a small brief brisk muscle contraction.
Muscle testing
To test motor function the examiner isolates a muscle associated with specific cervical and lumbar nerve roots and asks the patient to apply resistance to pressure. The muscle strength is graded from complete paralysis +0/5 to full strength +5/5. For complete instruction in the techniques and interpretation of manual muscle testing the reader should refer to a physical examination text such as Hoppenfeld’s (1976).
Clinical example
Dermatomal nerve pain due to disc injuries
Spinal nerve roots most commonly become painful because of exposure to the inflammatory chemicals released by the spinal discs. The central part of the disc, or nucleus pulposus, is rich in a highly inflammatory substance called phospholipase-A2 (PLA2). PLA2 has the ability to turn ordinary membranes into the cellular equivalent of battery acid, damages the nerve and eventually impairs its ability to conduct impulses (Ozaktay 1995, 1998). If the disc ruptures and releases a piece of nucleus material it is said to herniate. If the disc nucleus is injured but remains contained within the disc annulus it is said to be a contained herniation. If the herniated disc material forms a fragment and compresses the nerve it can create serious damage to the nerve including complete loss of sensory and motor function and compromise of deep tendon reflexes which may become permanent if not treated appropriately. A surgical consult is prudent if reflexes or muscle strength is lost even if the patient is to be treated with FSM.
It is possible for the disc to be minimally damaged with a small tear in the annulus that allows the PLA2 in the nucleus to leak out and create chemical inflammation in the nerve (Olmarker 1993, 1995). This has been called “chemical neuritis” (Marshall 1977). Inflammation in the posterior joints of the spine called facet joints can diffuse out to the nerve roots and create neuropathic pain and osteophytes from the facet joints can both inflame and mechanically compress dermatomal nerves. Any condition or pathology that creates an inflammatory response in the vicinity of the nerve can contribute to or cause neuropathic inflammation and pain.
Diagnosing dermatomal nerve pain due to nerve traction injuries
Treating dermatomal nerve pain
• The patient must be hydrated to benefit from microcurrent treatment.
• Hydrated means 1 to 2 quarts of water consumed in the 2 to 4 hours preceding treatment.
• Athletes and patients with more muscle mass seem to need more water than the average patient.
• The elderly tend to be chronically dehydrated and may need to hydrate for several days prior to treatment in addition to the water consumed on the day of treatment.
• DO NOT accept the statement, “I drink lots of water”
• ASK “How much water, and in what form, did you drink today before you came in?”
• Coffee, caffeinated tea, carbonated cola beverages do not count as water.
Channel B: tissue frequencies
• Dermatomal or Peripheral Nerve: ___ / 396
 Fascia is the thin connective tissue covering surrounding the muscles and virtually all visceral tissue. Nerves travel in a fascia–nerve–fascia sandwich. Any time a nerve is inflamed or injured the fascia becomes inflamed to some extent and adhesions will develop between the nerve and the fascia.
Fascia is the thin connective tissue covering surrounding the muscles and virtually all visceral tissue. Nerves travel in a fascia–nerve–fascia sandwich. Any time a nerve is inflamed or injured the fascia becomes inflamed to some extent and adhesions will develop between the nerve and the fascia. The well innervated disc annulus is the most pain sensitive and most easily injured portion of the disc. It consists of coiled layers of sturdy connective tissue wrapped around the gel like nucleus.
The well innervated disc annulus is the most pain sensitive and most easily injured portion of the disc. It consists of coiled layers of sturdy connective tissue wrapped around the gel like nucleus. The gel like disc nucleus fills the center of the disc and absorbs water to become a cushion for the vertebral bodies in the spine. It is very high in PLA2 and very inflammatory.
The gel like disc nucleus fills the center of the disc and absorbs water to become a cushion for the vertebral bodies in the spine. It is very high in PLA2 and very inflammatory.• Muscle Tissue as a tissue type: ___ / 46
 This frequency is rarely used and is thought to possibly represent the sarcomere or some contractile portion of the muscle. Combining the frequency to “increase secretions” in the nerve and “muscle tissue” has been observed to eliminate neuropathic atrophy in numerous cases. This protocol has no effect on disuse atrophy.
This frequency is rarely used and is thought to possibly represent the sarcomere or some contractile portion of the muscle. Combining the frequency to “increase secretions” in the nerve and “muscle tissue” has been observed to eliminate neuropathic atrophy in numerous cases. This protocol has no effect on disuse atrophy.Treatment protocol
Channel A condition / Channel B tissue
Reduce the pain
40 / 396
• Reduce inflammation / in the nerve
• 40 / 396 polarized positive is consistently effective in reducing nerve pain.
• Current polarized positive +
• Positive leads at the spine – Negative leads at the distal end of the nerve. See photos for details of set up.
• Treatment time. Use 40 / 396 until the pain has been reduced to approximately 2/10 on a 0 to 10 VAS scale. In most cases of dermatomal nerve pain this will take approximately 20–30 minutes but it may take up to an hour. Pain reduction usually begins distally and progresses proximally. If the patient is on narcotics or is dehydrated or has low essential fatty acid levels due to poor diet or lack of supplementation, response may be slow or poor. See the notes on what to do if response to treatment is slow.
• It is essential to reduce the pain by using 40 / 396 before treating the nerve for any other pathology.
Remove the basic pathologies / from the nerve
970, 94, 321, 9 / 396
• Remove the emotional component, remove nerve trauma, restore function, and remove histamine / from the nerve
• This sequence comes from Van Gelder’s concept of concussion as discussed in Chapter 10. In Van Gelder’s model, emotional shock and trauma leads to “paralysis” which leads to “allergy reaction” and reduction in secretions and vitality.
• “Emotional shock” from an injury or trauma changes tissue function; 970 / takes the “fact of” this emotional shock out of the membrane.
• “Trauma” stuns the nerve, overloading it rather like a power surge that trips a circuit breaker causing it to switch into something like a “safe mode.” The “safe mode” preserves the most important critical functions and allows for repair and recovery at some later time.
• “Paralysis” does not refer to complete loss of function or true medical paralysis. The analogy to the loss of function when a computer “locks up” and loses the ability to move to the next step is most apt. The fact of the trauma interferes with the smooth transfer of information within the tissue that allows it to know what to do next. The frequency to remove “paralysis” is Van Gelder’s conceptual equivalent of the computer command “control–alt–delete” that reboots the computer system.
• “Allergy Reaction” refers to the body’s first response to any dysfunction which is to release histamine as a way of starting the inflammatory cascade. Removing the allergy reaction allows the tissue to complete the return to normal function.
• Note: These four frequencies are known collectively as “The Basics”. They are usually used in sequential order as a group combined with the channel B frequency for the injured tissue but each can be used individually as needed based on the patient’s condition.
• Treatment time. Use each frequency for 1 to 2 minutes each. Current polarized positive.
Remove pathologies from the nerve
284 / 396
• Chronic Inflammation / Nerve
• Treatment time: Use 284 / 396 for 2 to 5 minutes after treating with 40 / 396 when the pain is down to a 2–3/10 and when the nerve pain has been present for longer than 3 months. Acute and chronic inflammation coexist and 284 / has a different but complementary affect on chronic nerve pain.
Soften the tissue
91 / 142
As it turns out, calcium ions flow into nerve and fascial membranes during nerve depolarization and inflammation (Winquist 2005). Using 91/396, 142 seems to remove the calcium influx that perpetuates nerve pain and hardens the fascia around the nerve although there are no biopsy findings to confirm this. Clinically these frequency combinations produce profound softening of the tissue and reductions in pain and the exact mechanism of these changes is yet to be discovered.
Treatment application
Current level
• 100–300μamps for the average healthy patient.
• Use lower current levels of 20–60μamps for very small or debilitated patients. Current levels above this will be irritating and may make the patient restless or agitated.
• Use higher current levels of 300–500μamps for larger or very muscular patients.
• In general, higher current levels reduce pain more quickly and improve response.
• Do not use more than 500μamps as animal studies suggest that current levels above 500μamps reduce ATP formation.
• Current Polarized Positive +: Current is polarized positive for most nerve treatments except for shingles (see Chapter 9) and peripheral neuropathies which require alternating DC current. Nerves respond very well and very quickly to polarized positive current.
• Waveslope: The waveslope refers to the rate of increase of current in the ramped square wave as it rises in alternating mode from zero up to the treatment current level every 2.5 seconds on the Precision Microcurrent and the automated family of FSM units. Other microcurrent instruments may have slightly different wave form choices. A sharp waveslope has a very steep leading edge on the square wave shape indicating a very sharp increase in current. A gentle waveslope has a very gradual leading edge on the waveform indicating a gradual increase in current.
Lead placement
• Positive leads. The positive leads contact wraps around the neck or is placed along the spine at the exiting nerve root. The positive contact must cover at least the foramenal area where the nerve exits the spine
• Negative leads. The negative leads attached to an adhesive electrode or wrapped in the warm wet fabric towel are placed at the end of the dermatome or nerve being treated. The placement is shown in the photographs.
• Electrode Contacts. FSM typically uses graphite gloves to conduct the current but any conductor can be used as long as it has low resistance and can be wrapped around the spine at the exiting nerve root and have good contact with the distal end of the nerve being treated.
• Adhesive electrode pads can be used if they are long enough (3 inches) to encircle the foramenal area where the nerve exits the spine and to give good contact at the distal end of the nerve.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree