Traumatic Shock
Christopher B. Colwell and Ernest E. Moore
Shock is the ultimate consequence of inadequate tissue perfusion, which may be manifested clinically by hemodynamic disturbances or organ dysfunction. At the cellular level, shock initially results from the insufficient delivery of required metabolic substrates, principally oxygen, to sustain aerobic metabolism. In the setting of trauma, shock is most often related to loss of circulating blood volume caused by hemorrhage, although inadequate oxygenation, cardiac dysfunction, neurologic dysfunction, or mechanical vascular obstruction may be either primary or contributing factors.
There are four general pathophysiologic types of shock: (a) hemorrhagic or hypovolemic, (b) cardiogenic, (c) neurogenic or vasogenic, and (d) septic. Hemorrhagic shock involves the loss of circulating intravascular volume caused by blood loss internally, externally, or both. Cardiogenic shock indicates a process that prevents the normal pumping of the heart and can be caused by pericardial tamponade that prevents normal ventricular filling, tension pneumothorax with vena caval compression and reduction in venous return to the heart, or direct cardiac damage with loss of contractile force (myocardial contusion).
Neurogenic or vasogenic shock may result from spinal cord injury with loss of peripheral vascular resistance. Major spinal cord injury results in acute vasodilatation but generally does not cause impaired tissue perfusion unless other injuries are present. Spinal cord injuries result in a loss of sympathetic tone and are, therefore, accompanied by bradycardia (spinal shock). Septic shock refers to a hyperdynamic response (elevated cardiac output and low systemic vascular resistance), followed by decreased cardiac output and increased systemic vascular resistance, eventually resulting in organ function deterioration associated with the inflammatory mediators accompanying infection.
If the acute stress of the traumatic shock state is sufficiently severe or prolonged, organ dysfunction may also develop, including acute kidney injury (AKI), adult respiratory distress syndrome (ARDS), and multiple organ failure (MOF). These entities can develop within a few hours to several weeks after the acute injury.
Shock is a common and potentially treatable cause of death in injured patients. Traumatic shock usually results from one or more of the following mechanisms: (a) acute blood loss from solid organ injury, rib fractures, major vascular injury, pelvic fracture, or multiple long-bone fractures; (b) cardiac dysfunction from blunt cardiac injury, tension pneumothorax, or pericardial tamponade; (c) hypoxemia caused by airway compromise or pulmonary contusion; or (d) loss of vasomotor integrity (distributive) caused by neurologic injury and late overwhelming infection.
The pathophysiology of traumatic shock is largely related to an imbalance in oxygen supply and demand. In the early phase of acute trauma, this imbalance is often caused by hypoperfusion, although low arterial blood oxygen saturation may be a significant contributing factor. Aggressive resuscitation with fluids having low or no oxygen-carrying capacity in the setting of severe and ongoing hemorrhage may lead to a critically low hemoglobin concentration that further contributes to tissue oxygen debt.
Acute blood loss elicits compensatory hemodynamic changes that serve to maintain vital organ perfusion. These changes include increased heart rate and vasoconstriction primarily in the peripheral circulation and splanchnic vascular beds. The term compensated shock refers to varying degrees of tachycardia and peripheral vasoconstriction that maintain adequate critical organ perfusion. Arterial blood pressure in compensated shock states may be moderately low or within the normal range. The term uncompensated shock indicates inadequate vital organ perfusion.
Vasoconstriction in response to acute blood loss is a protective response that preserves perfusion to the vital organs at the expense of peripheral and abdominal organ perfusion. In the hypoperfused tissues, oxygen demand exceeds oxygen delivery, and the numerous cellular processes that depend on oxidative metabolism and subsequent production of adenosine triphosphate (ATP) begin to fail. Acidosis results when aerobic metabolism slows, as protons cannot be used to synthesize ATP from adenosine diphosphate (ADP); lactate cannot be converted to pyruvate and, therefore, lactate increases. If the pathologic processes are not corrected, cellular dysfunction and eventually organ failure result.
CLINICAL PRESENTATION
Initial findings in a patient with traumatic shock may include tachycardia, hypotension, signs of poor peripheral perfusion, and alteration in mental status. In response to a loss of circulating volume, the heart rate will increase to sustain normal cardiac output. Continued blood loss will eventually result in a decrease in blood pressure. Peripheral vasoconstriction and central venoconstriction shunt blood centrally and result in a narrowed pulse pressure. Clinically, decreases in peripheral perfusion manifest as cool, pale, clammy extremities with prolongation of capillary refill (Table 20.1).
TABLE 20.1
Classes of Hemorrhagic Shock
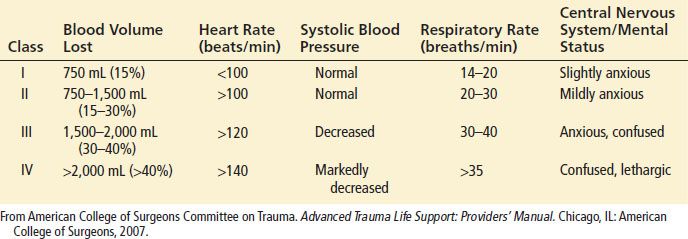
The skin may become mottled in appearance, but cyanosis is not a common finding. The narrowed pulse pressure can make the pulse quality weak or thready. Alterations in mental status caused by hypoperfusion may be subtle initially and can be difficult to distinguish from associated head injury or intoxication. Therefore, altered mental status on presentation or a subsequent decline in mental status, especially in patients without head trauma, should raise concern of systemic hypoperfusion and impending circulatory collapse.
Shock may be present even in the setting of “normal” vital signs. Young, healthy patients with substantial blood loss may maintain a blood pressure within the normal range by compensatory vasoconstriction and increased heart rate. Heart rate can occasionally be in the upper normal range. In such patients, signs of peripheral hypoperfusion and subtle changes in mental status may be the only warning signs preceding rapid hemodynamic decompensation. Elderly patients may not develop a tachycardic response to blood loss because of pre-existing medical conditions or medications. In fact, a bradycardic response may occur with rapid blood loss that is believed to be vagally mediated. Conversely, hypotension and tachycardia may be exacerbated in a pregnant trauma patient for a given degree of blood loss caused by compression of the inferior vena cava by the gravid uterus, resulting in decreased venous return.
Decline in urine output caused by renal hypoperfusion and renal fluid reabsorption is an important manifestation of shock physiology. Monitoring urine output may be useful in the emergency department (ED); later, it is a critical parameter in the surgical intensive care unit. Therefore, a urinary catheter should be placed in patients who exhibit evidence of shock, and the bladder should be drained to begin urine-output monitoring and to check for hematuria.
DIFFERENTIAL DIAGNOSIS
The most common cause of postinjury shock is hemorrhage-induced hypovolemia. However, several pathologic entities must be considered as either potential contributors or primary causes of shock. These entities are listed in Table 20.2.
TABLE 20.2
Causes of Shock

Pericardial tamponade is classically described as exhibiting the Beck triad of hypotension, distended neck veins, and muffled heart sounds. A pulsus paradoxus of >10 mm Hg may also be seen. However, not all of these markers may be present or detected. In a hypovolemic patient, the neck veins may be flat. Muffled heart sounds may be difficult to distinguish in a busy trauma resuscitation room, and detection of a pulsus paradoxus is not reliable. Pericardial tamponade should be considered whenever there is penetrating chest trauma, when there are rib fractures near the heart, or when there has been significant blunt trauma to the chest. The immediate availability of ultrasonography or echocardiography offers the best potential for rapid and accurate diagnosis.
A large pneumothorax or hemothorax can usually be detected by diminished breath sounds, although auscultation can be unreliable. Ultrasound (US) is useful for confirmation with a chest radiograph warranted if the US is equivocal. A tension pneumothorax may also result in deviation of the trachea away from the affected side, cardiac displacement, and hypotension related to inferior vena caval compression that limits venous return to the heart. However, tracheal deviation and cardiac displacement are late findings and may be difficult to detect by physical examination. Hypoxemia may be an earlier sign of a tension pneumothorax than hypotension. If a patient is hypotensive and has clinical evidence of a pneumothorax, placement of a chest tube before diagnostic imaging is appropriate.
Blunt cardiac injury (formally referred to as myocardial contusion) resulting in significant contractile dysfunction is a difficult diagnosis to make in the ED unless echocardiography is immediately available. Blunt cardiac injury should be suspected when blunt trauma involves the sternum and anterior left chest, particularly if ventricular ectopy, dysrhythmias, or electrocardiographic demonstration of ST-segment elevations (especially in the anterior precordial leads) are present. In addition, blunt trauma can result in valvular disruption and coronary artery dissection. Evidence of cardiogenic shock (hypotension, tachycardia, elevated central venous pressure) should alert the physician to the possible presence of a blunt cardiac injury or pericardial tamponade.
Spinal cord trauma with neurogenic shock may present with hypotension caused by loss of peripheral vascular resistance. In addition, because of the loss of sympathetic tone, these patients will not have the usual tachycardic response to hypotension but often demonstrate bradycardia instead. In this setting, the presence of neurologic deficits and lack of signs of peripheral vasoconstriction should arouse suspicion for this entity. Such patients generally have warm extremities and good urine output. Volume status must be carefully monitored, because excess fluid administration in patients with spinal shock may be detrimental.
Signs of shock in a trauma patient may not be a direct consequence of the injury. For example, a myocardial infarction may be the cause of a motor vehicle collision, or the physiologic stress associated with trauma may precipitate myocardial ischemia in a patient with underlying coronary artery disease. History, physical examination, or electrocardiographic findings consistent with myocardial infarction should arouse suspicion of combined medical and surgical processes. Another rare medical cause of hypotension in a trauma patient is anaphylaxis that may have preceded the trauma. Pharmacologic agents such as β-adrenergic blockers and recreational agents, such as ethanol and cocaine, may also significantly affect the clinical picture in the setting of acute trauma.
ED EVALUATION
The initial step in managing shock in the injured patient is prompt recognition. Effective management of the acutely injured patient exhibiting signs of shock requires that initial assessment and treatment begin simultaneously (1) with an emphasis on the airway, breathing, and circulation (ABCs). Assessment of airway patency, adequacy of ventilation (respiratory excursion and lung auscultation), hemodynamic status (pulse rate, central and peripheral pulse quality, blood pressure), and evidence of controllable hemorrhage should be immediately linked with interventions to (a) secure the airway while protecting the cervical spine, (b) enhance oxygenation, (c) provide ventilatory assistance, (d) limit further hemorrhage, (e) gain intravenous access, (f) initiate volume replacement, (g) obtain blood for laboratory and blood bank testing, and (h) perform US screening of the chest and abdomen (Fig. 20.1).
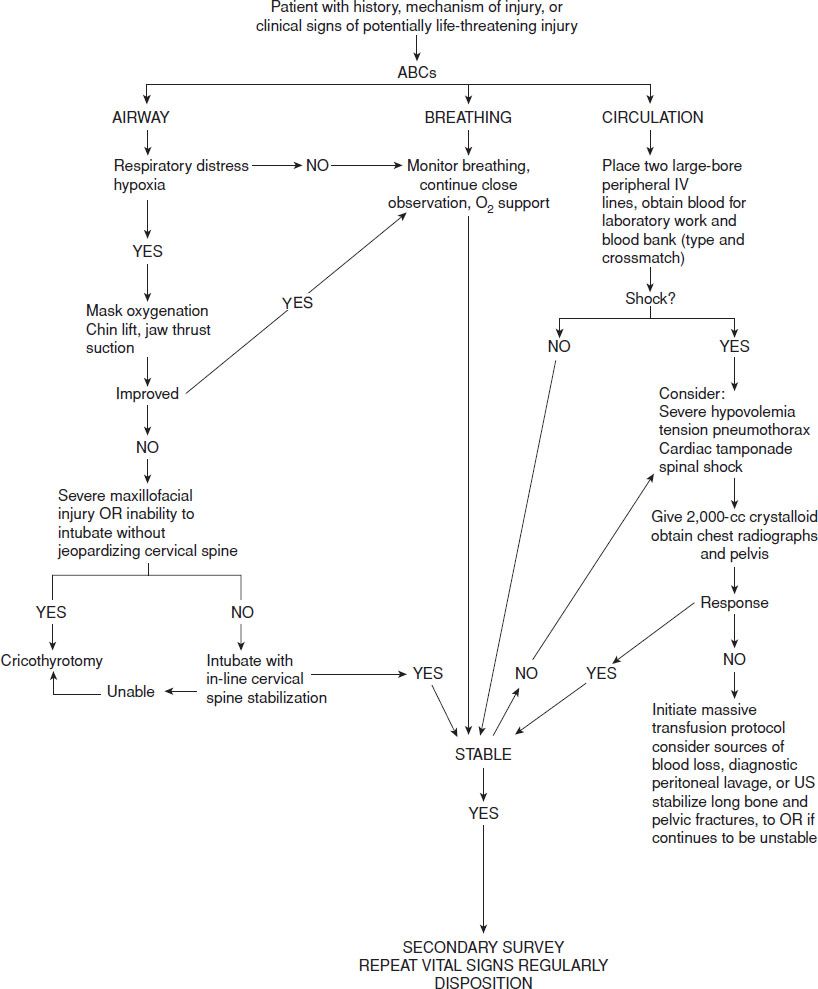
FIGURE 20.1 Treatment algorithm for traumatic shock.
After the primary assessment and initiation of treatment, a thorough secondary assessment should be conducted to identify potential injuries to the head, neck, chest, abdomen, pelvis, back, extremities, neurologic, and vascular system. Hypothermia can depress hemodynamics and aggravate coagulopathy and should be aggressively treated. Acute trauma is a highly dynamic disease process that requires frequent and careful reassessment of ventilation, hemodynamic status, and physical examination to optimally adjust therapeutic interventions and identify evolving pathologic processes.
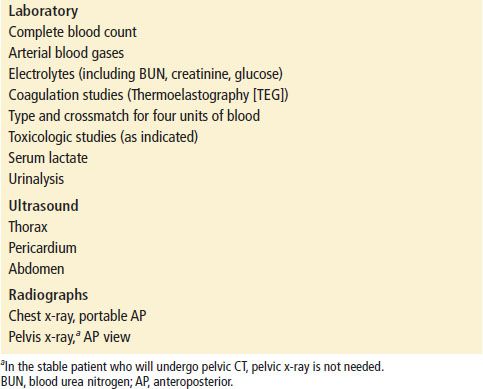
Initial laboratory studies in patients with major trauma should include (a) complete blood count (CBC); (b) arterial blood gases (base deficit); (c) electrolytes; (d) coagulation studies; (e) type and cross match for four units of packed red blood cells (PRBCs); and (f ) toxicologic studies (Table 20.3). Urine should be obtained and checked for blood. Base deficit is a reliable early index of the magnitude of shock (2). Serum lactate has been advocated by some as an early predictor of morbidity and mortality, particularly in burn patients (3), but it appears to lag behind base deficit in the first 12 hours after injury (4).
TABLE 20.3
Initial Laboratory and Radiographic Studies in Major Trauma