Transfusion Therapy
Michael R. King
Jonathan E. Charnin
I. THE DECISION TO INITIATE TRANSFUSION THERAPY
The decision to transfuse is complicated. There are many risks to transfusion (see section VIII), and transfusion is ultimately indicated when the potential benefits outweigh these risks. In most civilian settings, transfusion therapy utilized blood components rather than whole blood. The benefits of blood component transfusion stem from the correction of conditions that result in decreased production; increased utilization, destruction, or loss; or dysfunction of a specific blood component (red cells, platelets, or coagulation factors). Although many attempts at creating universal transfusion protocols have been introduced, few have successfully demonstrated definitive thresholds or clear superiority. Our aim in this chapter is to provide the background to help make these decisions on an individual basis.
A. Assessing Red Cell Mass and Oxygen-Carrying Ability
1. The decision to transfuse. Most evidence suggests that higher red cell transfusion thresholds (e.g., 10 to 12 g/dL) do not confer a mortality benefit and may even be harmful. Nonetheless, maintaining adequate red cell mass is essential for adequate oxygen-carrying capacity to the tissues. The decision to transfuse, therefore, must be based on a variety of patient and situational factors rather than rigid guidelines.
2. Patient factors. The main reason for transfusing red cells is to maintain oxygen-carrying capacity to the tissues, a primary determinant of which is the hemoglobin (Hb) level.
a. Healthy individuals or individuals with chronic anemia can usually tolerate Hb levels of 6.5 to 8 g/dL, assuming normal intravascular volume.
The use of a “restrictive transfusion practice,” aiming for Hb of 7 to 9 g/dL, is generally safe and may reduce the risk of death compared with transfusing toward a higher (10 to 12 g/dL) Hb goal. The reason for reduced mortality in the restrictively transfused group has not been clearly delineated, but the effects of allogeneic transfusion on diminished immune function have been substantiated in animal and human studies (see section VIII.D).
b. For patients with coronary artery disease, the risk of myocardial ischemia due to anemia has resulted in most practitioners transfusing to a higher (9 to 10 g/dL) Hb level. As the incidence of coronary artery disease rises substantially with age, many practitioners opt to use higher transfusion threshold in the elderly. Studies to support this practice, however, are lacking, and those that exist have produced contradictory results. A study of medical patients with acute coronary syndromes also found a higher mortality rate attributable to red cell transfusion in otherwise stable patients with a hematocrit (Hct) greater than 25%.
3. Situational factors
a. Intraoperative blood transfusion depends on red cell loss. This can be roughly estimated by measuring blood in suction canisters, weighing sponges, and checking blood loss in the drapes. In the perioperative period, the most common indication for transfusion is hemorrhage. During ongoing blood loss, it may be indicated to
transfuse even with a Hb greater than 10 g/dL if bleeding is brisk enough that the practitioner expects a substantial drop in the Hb in the absence of treatment.
transfuse even with a Hb greater than 10 g/dL if bleeding is brisk enough that the practitioner expects a substantial drop in the Hb in the absence of treatment.
b. If a patient is anemic preoperatively, the etiology should be clarified. It may be secondary to decreased production (marrow suppression or nutritional deficiencies), increased loss (hemorrhage), or destruction (hemolysis).
4. Estimating blood volumes (BVs)
a. Estimated allowable blood loss (EABL) can be calculated as follows, using either Hcts (as shown) or Hbs:
EABL = [(Hctstarting − Hctallowable) × BV]/[(Hctstarting + Hctallowable)/2]
BV in an adult is approximately 7% of lean body mass. This may be calculated as approximately 70 mL/kg of body weight in a normal adult man and approximately 65 mL/kg of body weight in a normal adult woman (see Chapter 31, for pediatric considerations). Obese patients have a lower percentage of body weight as blood, and the greater the degree of obesity, the lower the BV estimate on a per-kilo basis. With a body mass index (BMI) of 40, 53 mL/kg has been suggested. BV in patients with a BMI of 70 is estimated using 40 mL/kg of actual body weight.
b. Estimating the volume of blood to transfuse can be calculated as follows:
Volume to transfuse = [(Hctdesired − Hctpresent) × BV]/Hcttransfused blood
A unit of packed red blood cells (PRBCs) has a Hct of 70% to 85% using the Adsol preservative.
B. Thrombocytopenia is due to either decreased bone marrow production (e.g., chemotherapy, tumor infiltration, or alcoholism) or increased utilization or destruction (e.g., trauma or surgery creating a large wound, hypersplenism, idiopathic thrombocytopenia purpura [ITP], disseminated intravascular coagulation [DIC], or drug effects). It is also seen with the dilution and loss associated with massive blood transfusion (see section IX.A.1). Spontaneous bleeding is unusual with platelet counts above 20,000/mm3. Platelet counts above 50,000/mm3 are preferable for surgical hemostasis, with the caveat that, much like with RBCs, the decision to transfuse must be made based upon clinical factors rather than merely the platelet count. For example, patients with ITP maintain high platelet turnover and will often have normal coagulation despite a low platelet count. In addition, as the process lowering the platelet count is destructive, they will not respond well to platelet transfusion.
C. Coagulopathy. Bleeding associated with documented factor deficiencies or prolonged clotting studies (prothrombin time [PT] and partial thromboplastin time [PTT]) mandates replacement therapy to maintain normal coagulation function. See sections II and IX for a discussion of coagulopathy.
II. COAGULATION STUDIES
The most important clue to a clinically significant bleeding disorder in an otherwise healthy patient remains in the history. A history of anemia requiring iron replacement may suggest a bleeding tendency. Prior surgical bleeding, gingival bleeding, easy bruising, epistaxis, or menorrhagia should raise concern. Many tests are available to assess the coagulation system. However, the clinician must remember that the coagulation system is a complex interplay of platelets and coagulation factors.
A. Activated partial thromboplastin time (aPTT) is performed by adding particulate matter to a blood sample to activate the intrinsic coagulation system. Normal values for the PTT are 22 to 34 seconds, varying according to the reagent and instruments used by the specific laboratory. The aPTT assesses factors in the intrinsic (XI, XII, VIII, IX, and contact factors) and the common (II, V, X, and fibrinogen) coagulation pathways. The test is sensitive to decreased amounts of coagulation factors and is elevated in patients on heparin therapy. The aPTT will be abnormal in patients who have hemophilia or a circulating anticoagulant (e.g., lupus anticoagulant or antibodies to factor VIII). The clinician should remember that an abnormal aPTT does not necessarily correlate with clinical bleeding. Aggressive correction of an abnormal aPTT in surgical patients is not always indicated, unless the patient is actively bleeding.
B. Prothrombin time is a measure of factors of the extrinsic (VII and tissue factor) and common (see above) coagulation pathways and is measured by adding tissue factor to a blood sample. While both PT and aPTT are affected by levels of factors V and X, prothrombin, and fibrinogen, the PT is specifically sensitive to deficiencies of factor VII. The PT is normal in deficiencies of factors VIII, IX, XI, XII, prekallikrein, and high molecular weight kininogen.
C. The international normalized ratio (INR) is a means of standardizing PT values to allow comparisons among different laboratories or at different times. It is the ratio of the patient’s PT to the control PT value that would be obtained if international reference reagents had been used to perform the test. Oral warfarin anticoagulation therapy may therefore be guided by a target INR value that is independent of the laboratory variability of the PT. For example, an INR of 2.0 to 3.0 is recommended for prophylaxis against thromboembolism in atrial fibrillation.
D. Activated clotting time (ACT) is a modified whole blood clotting time in which diatomaceous earth (celite) or clay (kaolin) is added to a blood sample to activate the intrinsic clotting system. The ACT is the time until clot formation. A normal ACT is 90 to 130 seconds, depending on the instrument used. The ACT is a relatively easy and expedient test to perform and is useful in monitoring heparin therapy in the operating room (see Chapter 24). Because the ACT is relatively insensitive to lower levels of heparin anticoagulation, it is typically reserved for settings that require full anticoagulation such as cardiopulmonary bypass and extracorporeal membrane oxygenation (ECMO).
E. Fibrinogen is the precursor protein that is dimerized to form the strong fibrin components of clots. Assaying fibrinogen levels can be helpful when coagulopathy is present or suspected. While the diagnostic specificity of the fibrinogen level is low, it can be helpful to guide further transfusion support (i.e., the administration of FFP or cryoprecipitate).
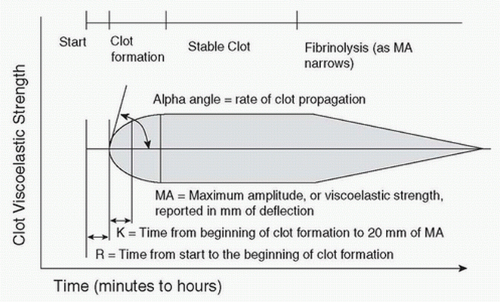
F. Thromboelastography (TEG) is available in some centers for clinical use, often as a point-of-care test. TEG is performed by placing a small aliquot of blood into a heated oscillating cup into which is suspended a pin on a torsion wire. Clot formation in the oscillating cup generates torque on the pin, and the torque is measured and converted to an electrical signal. The signal is recorded by a computer, creating a characteristic trace (Fig. 35.1) that may be analyzed for abnormalities of clot formation. By measuring clot formation and viscoelastic clot strength, TEG provides information about the adequacy of clotting factors, fibrin levels, and platelets.
In general, R represents the activity of coagulation factors, maximum amplitude the platelet function/number, and the alpha angle the acceleration of fibrin formation. Thromboelastometry or ROTEM uses a similar technology, with similarly measured parameters.
In general, R represents the activity of coagulation factors, maximum amplitude the platelet function/number, and the alpha angle the acceleration of fibrin formation. Thromboelastometry or ROTEM uses a similar technology, with similarly measured parameters.
III. BLOOD TYPING AND CROSS-MATCHING
A. Donor blood and recipient blood are typed using the red cell surface ABO and Rh systems and screened for antibodies to other cell antigens. “Direct” cross-matching involves directly mixing the patient’s plasma with the donor’s red cells to establish that hemolysis does not occur from any undetected antibodies. An individual’s red cells have A, B, AB, or no surface antigens, which is then designated type O. If the patient’s red cells are lacking either surface antigen A or surface antigen B, then antibodies will be produced against it. A person who is type B will have anti-A antibodies in the serum, and a type O individual will have circulating anti-A and anti-B antibodies. Consequently, a person who is type AB will not have antibodies to either A or B and can receive red blood cells (RBCs) from any blood type. Type O blood has neither A nor B surface antigens and can donate blood cells to any other type (universal red cell donor; Table 35.1).
The universal FFP donor, conversely, is AB because it will contain neither anti-A nor anti-B antibodies. Whole blood donors and recipients must be exact ABO matches because whole blood contains both RBCs and serum. For example, a unit of type O whole blood will contain serum with anti-A and anti-B antibodies and therefore cannot be used to transfuse patients who are A, B, or AB blood types.
The universal FFP donor, conversely, is AB because it will contain neither anti-A nor anti-B antibodies. Whole blood donors and recipients must be exact ABO matches because whole blood contains both RBCs and serum. For example, a unit of type O whole blood will contain serum with anti-A and anti-B antibodies and therefore cannot be used to transfuse patients who are A, B, or AB blood types.
TABLE 35.1 Transfusion Compatibility | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
B. Rh surface antigens are either present (Rh-positive) or absent (Rh-negative). Individuals who are Rh negative will develop antibodies to the Rh factor when exposed to Rh-positive blood. This is not a problem with the initial exposure, but with subsequent exposures, hemolysis will occur due to the circulating antibodies. This can be a particular problem during pregnancy. The anti-Rh antibodies are IgG and freely cross the placenta. Rh-negative mothers who have developed Rh antibodies will transmit these antibodies to the fetus. If the fetus is Rh positive, massive hemolysis will occur. RHO immune globulin, an Rh-blocking antibody, prevents the Rh-negative patient from developing anti-Rh antibodies. It should be administered to Rh-negative individuals who receive Rh-positive blood and to Rh-negative mothers delivering Rh-positive babies (some fetal maternal blood mixing occurs at delivery). The recommended dose is 300 µg intramuscularly for every 15 mL of Rh-positive blood transfused.
C. Recipient antibodies against other donor RBC antigens (most frequently those from the Kell, Kid, Duffy, or Lewis groups) can cause hemolytic transfusion reactions. When a patient’s blood sample is screened and found to have antibodies against antigens found on donor RBCs, it complicates cross-matching and can delay the availability of blood products. When a patient’s antibody screen is positive, it is advisable to discuss the anticipated transfusion needs with the blood bank.
D. If an emergency blood transfusion is needed, type-specific (ABO) red cells usually can be obtained within minutes if the patient’s blood type is known. If typespecific blood is unavailable, type O Rh-negative red cells should be transfused (type O-positive blood can be used emergently in males). Type-specific blood should be substituted as soon as possible to minimize the amount of type O plasma (containing anti-A and anti-B antibodies) transfused.
IV. BLOOD COMPONENT THERAPY
A. General Considerations
1. One unit of PRBCs (Hct about 70% and volume about 250 mL) usually will increase the Hct by 2% to 3% or the Hb by 1 g/dL in an euvolemic adult once equilibration has taken place. PRBCs must be ABO compatible to the recipient.
2. One unit of platelets increases the platelet count by 5,000 to 10,000/mm3. A usual platelet transfusion is 1 unit per 10 kg of body weight. If thrombocytopenia is caused by increased destruction (due to development of antiplatelet antibodies) or if platelets are dysfunctional, platelet transfusions will be less efficacious. Transfusion of ABO-compatible platelets is not obligatory, although they may provide a better posttransfusion platelet count. Single-donor or HLA-matched platelets may be required for patients with a refractory response to platelet transfusion. A unit of single-donor platelets provides the equivalent of approximately six random donor units of platelets. Platelets should never be stored in a cooler.
3. Fresh frozen plasma (FFP, about 250 to 300 mL per unit), in a dose of 10 to 15 mL/kg, generally will increase plasma coagulation factors to 30% of normal, the minimal level necessary for hemostasis (excepting fibrinogen where 50% of the normal 200 to 400 mg/dL is required).
Fibrinogen levels increase by 1 mg/dL/mL of plasma transfused. Acute reversal of warfarin is often achieved with only 5 to 8 mL/kg of FFP, although the PT may remain modestly prolonged. FFP transfusions must be ABO compatible, but Rh compatibility and cross-matching are not required (Table 35.1).
Fibrinogen levels increase by 1 mg/dL/mL of plasma transfused. Acute reversal of warfarin is often achieved with only 5 to 8 mL/kg of FFP, although the PT may remain modestly prolonged. FFP transfusions must be ABO compatible, but Rh compatibility and cross-matching are not required (Table 35.1).
4. Cryoprecipitate is prepared from FFP and contains concentrated factor VIII, factor XIII, fibrinogen, von Willebrand factor (vWF), and fibronectin. Indications for cryoprecipitate include hypofibrinogenemia, von Willebrand disease, hemophilia A (when factor VIII is unavailable), and preparation of fibrin glue. Dosage is 1 unit per 7 to 10 kg, which will raise the plasma fibrinogen by about 50 mg/dL in a patient without massive bleeding. ABO compatibility is not mandatory for cryoprecipitate transfusion.
B. Technical Considerations
1. Compatible infusions. Blood products should not be infused with 5% dextrose solutions, because they cause hemolysis. Recent studies have suggested that coinfusion with lactated Ringer’s, which contains calcium may not induce clot formation, but concern persists. Coadministration of blood products with Plasmalyte solutions is probably safe. Normal saline (0.9%) solution, albumin (5%), and FFP are compatible with RBCs.
2. Blood filters. Standard blood filters (170 to 200 µm) remove debris and should be used for all blood components.
a. Leukocyte reduction is achieved by filtration, either in the blood bank or at the bedside. Microaggregate filters (20 to 50 µm), which should not be used for platelets, remove 70% to 90% of leukocytes. Thirdgeneration or adhesion filters remove more than 99.9% of leukocytes via a combination of filtration and adhesion of white blood cells. These filters are recommended for use in patients with a history of febrile, nonhemolytic transfusion reactions; for prevention of alloimmunization to foreign leukocyte antigens (e.g., in the oncologic patient expected to require multiple platelet transfusions); or to prevent cytomegalovirus (CMV) transmission in organ transplant recipients. Other potential but as yet unproved benefits of leukocyte reduction include a diminished immunomodulatory effect of allogeneic transfusion; reduced transmission of bacterial, viral, or prion diseases; prevention of transfusion-related acute lung injury (TRALI); and decreased incidence of graft versus host disease (GVHD). Several countries have implemented universal leukoreduction of transfused cellular blood products. The proposed benefits and cost-efficiency of universal leukoreduction are a matter of significant controversy within transfusion medicine and are not mandatory in the United States at this time.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree