Fig. 12.1
Schematic representation of the coagulation cascade demonstrating the interplay between coagulation, anticoagulation, clot formation and fibrinolysis. Grey boxes with red text indicate which components of the cascade are measured by that particular test; dashed red arrows indicate an inhibitory effect
12.3.1 Haemorrhagic Phenotype of Liver Cirrhosis
The bleeding diathesis is the phenotype that most clinicians in the ICU will be familiar with. Variceal bleeding is severe and life threatening, often resulting in massive transfusion. There is a long-standing dogma that patients with cirrhosis are “auto-anticoagulated”, based not only on an abnormal prothrombin time but also on the frequent occurrence of bleeding. Bleeding can range from trivial to life threatening including cutaneous, gingival or variceal bleeding and also menorrhagia or epistaxis. Factors contributing to bleeding can be considered in terms of defects in pathways of the coagulation cascade and other factors. Cirrhosis is characterised by a reduction in synthesis of all procoagulant factors, the exception being the powerful procoagulant factor VIII, which shows the opposite trend and is also synthesized in extrahepatic sites including the pulmonary vascular endothelium [21]. Platelet levels are important initiators of coagulations since their surface phospholipids provide the platform for assembly of coagulation factors. Whilst platelet numbers and function may be reduced, there is some evidence that platelet hyperactivation may compensate for this [22], perhaps in part due to increased circulating platelet-derived procoagulant microvesicles [23, 24] but also due to elevated levels of the platelet adhesion protein von Willebrand factor and reduced levels of its cleaving protease ADAMTS13 [25]. Finally, anaemia and excessive fibrinolysis may also impair response to bleeding or promote it.
Other factors which promote bleeding include sepsis, endotoxaemia and uraemia [14]. Infection is known to precipitate bleeding and use of antibiotics following variceal haemorrhage has been shown both to reduce severity of bleeding and early rebleeding in randomised trials [26, 27]. Infection has been associated with increased circulating heparinoids in patients with cirrhosis and active bleeding, which may indirectly interfere with coagulation [28]. Uraemia, which may be a result of concomitant renal impairment in patients with cirrhosis, may also contribute to a bleeding tendency due to abnormal platelet-vessel wall interactions and platelet dysfunction [29, 30].
12.3.2 Propensity Towards Thrombosis in Liver Cirrhosis
Thromboses in the portal and mesenteric vasculature are not uncommon in cirrhosis [31] and are thought to be mainly due to sluggish blood flow through the liver due to intrahepatic portal hypertension. However, recent population-based studies have described an increased risk of peripheral venous thrombotic events such as deep vein thrombosis and pulmonary emboli in patients with cirrhosis [32–34], a phenotype which is less recognized by most clinicians. Aside from derangements in the coagulation cascade, other recognised factors may contribute towards the risk of thromboembolism including immobility, hospitalisation and increasing age. Occurrence of an increased risk of these macro-thrombotic complications in cirrhosis is counterintuitive, due to the haemorrhagic complications observed in clinical practice, as well as conventional coagulation indices suggesting hypo-coagulation. This paradox may be better appreciated with an understanding of the tests used to measure deranged coagulation. Conventionally, assessment of coagulation status is based upon evaluation of the prothrombin time (PT), which is only sensitive to thrombin generation with respect to procoagulant factors and is insensitive to the inhibition of thrombin by anticoagulant factors [35]. Therefore, it is not surprising that the PT does not predict the risk of bleeding from varices, nor after invasive procedures, in patients with cirrhosis [36–38], but nonetheless is routinely used to guide transfusion.
More accurate global tests of coagulation include thrombin generation assays and thromboelastography/thromboelastometry, which have shown that plasma from patients with stable (non-bleeding) cirrhosis appears to be normal – hypercoagulable in comparison to healthy controls [16, 39], leading to the concept of “rebalanced haemostasis” [40], albeit a balance which is precarious and may lead to bleeding, thrombosis or even both (Fig. 12.2).
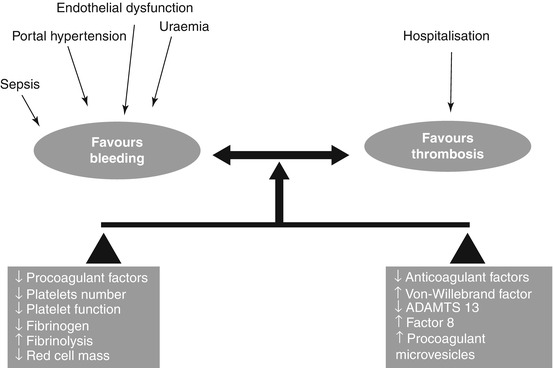

Fig. 12.2
Factors promoting bleeding and thrombosis in liver cirrhosis. The haemostatic balance in liver cirrhosis is precarious and can be easily tipped into a bleeding or thrombotic tendency with an appropriate trigger (Reproduced from Gut, Jairath and Burroughs, volume 62 (4), pages 479–82 with permission from BMJ publishing Ltd)
12.4 The Practice of Transfusion of Blood Components for Variceal Bleeding
There are no specific clinical guidelines for the transfusion management of variceal bleeding in cirrhosis. Indeed expert consensus guidelines for the management of variceal bleeding (Baveno guidelines) refrain from making any recommendations on transfusion thresholds given the lack of an evidence base. Two large epidemiological surveys from the UK have characterised actual transfusion practice for AVH.
12.4.1 UK National Audit of the Use of Blood Components in Acute Upper GI Bleeding
In this large nationwide audit, the majority of patients were transfused with RBCs (73 %), and the median haemoglobin (Hb) prior to transfusion was 8.1 g/dL (range 6.9–9.3) with a median of 4 units transfused in total (range 3–6). There was considerable practice variation; for example, in patients with a presenting of Hb >8 g/dL and no evidence of haemodynamic shock, 35 % were transfused with RBCs within 12 h of presentation; conversely 37 % of patients with a presenting Hb > 8 g/dL with evidence of haemodynamic shock were not transfused with RBCs. Fresh frozen plasma (FFP) was transfused in 35 % of patients with a median of 4 units per patient (range 2–6 units); the median INR prior to transfusion of FFP was 1.8 (1.3–2.1), and the median prothrombin time (PT) prior to transfusion was 20 (range 16–22). Seventeen per cent were transfused with FFP in the presence of a normal coagulation screen (INR ≤1.5 and/or PT < 3 s prolonged), whereas 41 % with an abnormal coagulation screen (INR > 1.5 and/or PT < 3 s prolonged) were not transfused with FFP. Platelets were transfused to 14 % (76/526) of presentations who were prescribed a median of 2 pools of platelets with a median platelet count prior to transfusion of 43 (IQR 30–62). Of those receiving platelet transfusion, 38 % (29/76) had a platelet count of >50. Overall, 7 % were transfused with cryoprecipitate.
12.4.2 UK National Audit of the Use of Blood Components in Liver Cirrhosis
This national project was undertaken in 2013 to establish patterns of use of blood components in liver cirrhosis. Data was collated on 1,313 consecutive admissions with cirrhosis from 85 hospitals recording all transfusions, whether administered for prophylaxis or treatment of bleeding. In the 192 cases of a variceal bleed, a remarkably similar pattern of transfusion was observed in comparison to the earlier audit in 2007; 92 % received RBCs, and in 54 % of those transfused, the presenting Hb was >7 g/dL. FFP was transfused in 32 % cases, and the pre-transfusion INR was <1.5 in 40 % of cases. Platelets were transfused to 14 % patients, and 46 % of cases had a platelet count of >50 × 109. Cryoprecipitate was transfused to 4 % cases. No patients were administered recombinant factor VII, and <5 % received antifibrinolytics.
12.5 Efficacy of Red Blood Cell Transfusion for Gastrointestinal Bleeding
12.5.1 Purpose of RBC Transfusion
During acute bleeding the priority is prompt restoration of circulating volume with intravenous fluids and RBCs to maintain regional and global tissue oxygenation, followed by definitive therapies to control bleeding and to prevent rebleeding (endoscopic, pharmacological and occasionally surgery/radiology). There is limited evidence available on which types of patients benefit from RBCs after AUGIB, at what threshold they should receive them and how much they should receive [41]. Most presentations with AUGIB do not have major bleeding or even features of haemodynamic compromise [42]. Furthermore, 70–80 % cases of AUGIB, especially non-variceal UGIB, will cease spontaneously without the need for endoscopic intervention [43, 44]. Hence, in the majority of cases, RBCs are transfused because the Hb has fallen below a threshold at which the risk of anaemia is perceived to outweigh the risks of transfusion. This threshold is influenced by many factors including the desire to have a “safe” Hb level in the event of rebleeding, to reduce symptoms of anaemia after bleeding has arrested, or by patient-related factors [45]. There is considerable practice variation with respect to RBC transfusion following AUGIB, with rates of transfusion ranging from 23 to 84 % across 208 hospitals in the UK [42].
12.5.2 Guidelines for RBC Transfusion in AUGIB
The evidence base to inform the transfusion recommendations in AUGIB guidelines is sparse and has largely arisen from rodent studies, observational studies and a small number of randomised controlled trials [46]. International consensus guidelines on the management of patients with non-variceal upper GI bleeding advocate a restrictive approach to transfusion using an Hb threshold of ≤7 g/dL [47], although this recommendation was based upon trials only conducted in critically ill patient cohorts, where acute bleeding was specifically excluded. Transfusion requirements may be reasonably expected to differ after acute bleeding due to rapid anaemia, haemodynamic compromise and lower Hb levels. Of particular concern in extrapolating this for AUGIB is the high burden of comorbidity, specifically overt and occult cardiovascular and ischaemic heart disease [7]. For patients with cirrhosis and bleeding secondary to variceal bleeding and portal hypertension, guidelines recommend maintaining the Hb around 8 g/dL [48].
12.5.3 Observational Studies on RBC Transfusion in AUGIB
The largest observational study of actual transfusion practice for AUGIB (non-variceal and variceal) to date comes from the UK audit of AUGIB in 2007 which showed practice variation as well as some evidence of inappropriate transfusion practice. An analysis of 4,441 patients who underwent in-patient endoscopy found that after statistical adjustment for markers of disease severity, RBC transfusion in patients with an Hb > 8 g/dL was associated with a twofold increased risk of further bleeding [49]. Similar results were reported in a Canadian study [50]. Whilst these observational studies suggest an association between RBC transfusion and adverse outcome after AUGIB (e.g. further bleeding), they do not prove a causal relationship, since there is a high likelihood of residual confounding in the analyses that arises due to more liberal administration of RBC to sicker patients. However, they provide an important basis to further develop the evidence base for the use of RBCs in AUGIB, by providing data to inform the design of randomised controlled trials (RCTs) of differing transfusion strategies to confirm or refute the findings of these observational studies [51].
12.5.4 Randomised Trial Evidence
A small trial conducted in the UK in 1986 was the first to suggest a causal link between early RBC transfusion after AUGIB and an increased risk of rebleeding, which the authors postulated was the result of transfusion reversing the hypercoagulable response to haemorrhage [52]. A Cochrane review of RCTs identified only three small trials in total comparing differing RBC transfusion strategies in patients with AUGIB from which no firm conclusions could be made [46].
A seminal trial of restrictive versus liberal RBC transfusion for AUGIB was published in 2013 [53]. This was conducted in a single-centre specialised bleeding unit in Spain and randomised over 900 patients into a trial of restrictive (transfusion when Hb <7 g/dL) versus liberal (transfusion when Hb <9 g/dL) RBC transfusion strategies. There were notable exclusion criteria including patients with a history of acute coronary syndrome, peripheral vascular disease, stroke or transient ischaemic attack. There were strict protocols of care within the trial in terms of the use and timing of endoscopy (all patients received endoscopy within 6 h of presentation), pharmacological therapies, administration of single units of RBCs and frequency of haemoglobin concentration measurements [51, 53]. The rates of further bleeding were significantly lower in the restrictive transfusion arm (10 % vs. 16 %), and mortality at 45 days was also lower in the restrictive transfusion arm (5 % vs. 9 %). The treatment effect for mortality was only significant for patients with milder liver cirrhosis and not for patients with peptic ulcer bleeding (which formed almost 50 % of patients in the trial). The rate of overall adverse events was greater in the liberal transfusion arm (48 % vs. 40 %), the key differences being an increase transfusion-associated circulatory overload (TACO) and transfusion reactions in the liberal arm.
This trial has provided a causal relationship between more liberal RBC transfusion after AUGIB and further bleeding and especially produces RCT evidence for the first time to support the age-old dogma of not “overtransfusing” variceal bleeds. However, caution must be exercised in generalising the findings of these results to all patients with AUGIB. Firstly, the stringent processes of care in the trial should be considered when extrapolating the results to each clinician’s own institution, especially the ability to provide therapeutic endoscopy within 6 h for all patients no matter what time or day they are admitted, since the availability of such an intervention may in turn influence transfusion thresholds. Secondly, the trial excluded patients with a history of ischaemic heart disease, vascular disease or stroke; given AUGIB is predominantly a disease of older patients, this would exclude almost 40 % of patients presenting in the UK based upon data from the UK audit in 2007 [54]. The optimum transfusion strategy for this group of patients at especially high risk of mortality remains unclear, and therefore a transfusion threshold of 7 g/dL cannot be advocated without further evidence [51]. Recent UK national institute for clinical excellence (NICE) guidelines for AUGIB acknowledge that further evidence is needed before universally extrapolating restrictive transfusion to all patients with AUGIB.
12.6 Mechanisms of Harm of RBCs for AUGIB
There are many postulated general mechanisms of harm associated with RBC transfusion including transfusion-related immunomodulation and adverse effects associated with ageing RBCs, which also apply to the patient with AUGIB. For patients with cirrhosis and portal hypertensive bleeding, the trial from Barcelona demonstrated in a subgroup of patients that more liberal transfusion was associated with elevated portal pressures, which is a plausible mechanism for both worsening bleeding and promoting rebleeding [53]. This does not explain the mechanism in the vast majority of in the trial who did not have liver cirrhosis. They also postulated that more liberal transfusion may interfere with coagulation to precipitate further bleeding, although this seems implausible with just a mean of two extra RBC units transfused in the liberal transfusion group. The mechanism of increased mortality in the liberal transfusion arm is most likely mediated through further bleeding, since this event is a strong and independent predictor of death [51].
12.7 Platelet Transfusions in GI Bleeding
Thrombocytopenia per se is common in critically ill patients and found to be a risk factor for bleeding and mortality. The prevalence of mild (<150 × 10−9/L) and moderate (<50 × 10−9/L) thrombocytopenia in adult patients on intensive care units is reported at 40 and 8 %, respectively [55]. Most evidence for the use of platelet transfusions comes from the use of prophylactic transfusion in haematological malignancy. Platelet transfusions are infrequently administered to patients with AUGIB; in the UK audit of AUGIB, just 3 % of all presentations to UK hospitals with AUGIB received platelet transfusion. Several consensus guidelines recommend platelet transfusion below a threshold of 50 × 109 in actively bleeding patients, although the evidence to support this is poor. In the UK audit, 61 % of patients who were actively bleeding with a platelet count of <50 × 109 did not receive a platelet transfusion, and 42 % of all platelet transfusions were administered to patients with a platelet count of >50 × 109 [42]. This variation and uncertainty in practice is likely to reflect the lack of an evidence base to inform the efficacious use of platelet transfusions for the management of AUGIB. There are special considerations for the use of platelets in patients with AUGIB.
12.7.1 Thrombocytopenia in Cirrhosis and Variceal Bleeding
Thrombocytopenia in patients with liver cirrhosis is due to three main reasons: (1) reduced production (as a result of decreased plasma thrombopoetin levels), (2) platelet sequestration (3) and accelerated platelet turnover. In addition to the absolute reduction in platelet count, there is likely to be a degree of thrombocytopathy due to abnormalities of the platelet glycoprotein Ib and defective synthesis of thromboxane A2 [56]. Despite both thrombocytopenia and thrombocytopathy, there is some evidence that highly elevated levels of von Willebrand factor in patients with liver cirrhosis contribute to primary haemostasis under experimental flow conditions and it is possible that this mechanism may compensate for defects in platelet number and function in cirrhosis [57]. A previous study of critically ill patients with cirrhosis found that platelet transfusion was the only component to significantly improve thromboelastography parameters [58], which provides some rationale for an interventional study. To date, no clinical studies have addressed whether administration of platelet transfusion to the actively bleeding patient with cirrhosis promotes haemostasis. Transfusion of any components should be judicious due to the risk of further exacerbating portal pressure.
12.7.2 Managing Antiplatelet Therapy in Non-variceal Bleeding
Over 40 % of patients are taking either an antiplatelet or NSAID agent prior to hospitalisation with non-variceal GI bleeding [54]. Given the burden of cardiovascular comorbidity, a proportion of patients who develop non-variceal bleeding are taking aspirin and/or clopidogrel or other antiplatelets. The effect of these drugs lasts for the duration of the platelet life span (i.e. 7–10 days), and the degree of platelet inhibition is affected by other factors such as genetic polymorphisms. There is little information in consensus guidelines to inform interventions to manage bleeding in patients taking antiplatelets. In one pilot study in healthy volunteers, transfusion of two autologous platelet components overcame clopidogrel-induced platelet reactivity, but not ADP-induced platelet aggregation. At present there is no evidence to support the use of platelet transfusions to patients taking antiplatelet agents who present with major gastrointestinal bleeding.
12.8 Plasma Transfusion in GI Bleeding
Almost half of fresh frozen plasma (FFP) transfused in the UK is to critically ill patients [59]. A large UK survey showed that 13 % of critically ill adult patients received FFP during an intensive care admission, and half of these were for the treatment of bleeding [59]. In theory, FFP may be a useful intervention for reduction of bleeding severity by improving deranged coagulation by replenishing procoagulant and antifibrinolytic factors lost through acute bleeding by providing a source of fibrinogen to promote haemostasis. In real-life practice, FFP is frequently transfused to patients with cirrhosis and variceal bleeding as part of initial resuscitation in response to abnormal standard laboratory coagulation indices on the assumption that patients have impaired procoagulant function [42, 53]. In stable cirrhosis, prolongation of the PT is rarely associated with abnormal global assays of coagulation such as thrombin generation [TG] or thromboelastometry [ROTEM] [40, 60], but it is not clear whether a similar discrepancy exists in the setting of acute haemorrhage. Many clinicians prescribe early FFP to correct assumed bleeding tendency, but the risk-benefit balance between haemostatic correction and potential hypervolaemia is uncertain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





