Tetanus is a potentially fatal disease characterized by hypertonia, muscle spasms, and autonomic instability. Tetanus is

caused by the action of tetanospasmin (commonly called
tetanus toxin), a potent neurotoxin elaborated by the organism
Clostridium tetani (
C. tetani).
Tetanus was known to Egyptians over 3000 years ago. Hippocrates gave the first detailed description of the disease in 400 BC, Arthur Nicolaier discovered the tetanus bacterium in 1884, and in 1889 Shibasaburo Kitasato at Koch’s Institute obtained the first pure culture of tetanus bacilli. German bacteriologist Emil Von Behring developed a toxin-antitoxin mixture that was an effective vaccine against tetanus. In 1893, Emile Roux, assistant to Louis Pasteur, improved procedures for using serum antitoxin to prevent and to treat tetanus. Tetanus antitoxin was first used during World War I, dramatically reducing the incidence of the disease.
Epidemiology
Tetanus remains a major world health problem despite the availability of active and passive immunization. The World Health Organization (
WHO) estimated in 2011 that the number of tetanus-related deaths globally in children <5 years of age was 72,600, of which 61,000 were attributable to neonatal tetanus (
1). Worldwide, tetanus affects all age groups, particularly newborns (who account for half of all cases) and children. Lack of seroprotective immunity against tetanus is common among children. Only 45% of children attending an emergency unit of a tertiary care hospital in Africa had seroprotection (
2). Tetanus rarely occurs among children who have received the primary series of tetanus toxoid vaccine. In industrialized nations such as the United States, tetanus is a disease of the elderly; a population that was either born before immunization programs were implemented or have an agerelated decline in antitoxin levels. Fewer than 75% of adults were immune or partially immune to tetanus in a serosurvey in Australia in 2005 (
3).
The Pathogen
Tetanus is caused by C. tetani, a drumstick-shaped, anaerobic, Gram-positive bacillus that forms endospores on maturation. The spores are widely distributed (soil, house and operating room dust, freshwater, and saltwater) and may survive for years. They are also present in the feces of a number of animals (sheep, cattle, dogs, cats, chickens, and horses) and in the intestinal tract of as many as 40% of humans. Soil rich in organic matter or treated with animal manure can be highly infective. The spores are resistant to extremes of temperature, moisture, various disinfectants (ethanol, phenol, and, formalin), and to boiling for 20 minutes.
Pathogenesis
Disease is initiated when
C. tetani spores enter a breach in the skin or mucosa. It typically occurs after acute injury to soft tissue, particularly deep penetrating or puncture wounds and lacerations, in which anaerobic bacterial growth is facilitated. The common portals of infection are wounds on the lower limbs, nonsterile intramuscular (
IM) injections, and compound fractures. Tetanus can also occur as a result of animal bites, drug injection with dirty needles, dental abscesses, body piercing, drug abuse (notably skin popping), burns, surgical procedures, and in patients with chronic infections, such as otitis media or decubitus ulcers. The portal of entry is not apparent in approximately one-third of patients.
Inside the wound, in an anaerobic environment, the spores transform into vegetative forms and proliferate. The process is facilitated by the presence of a foreign body, necrotic tissue, or suppuration in the wound. Replicating bacteria do not cause inflammation, and the wound appears benign. The vegetative forms produce two toxins under plasmid control: tetanospasmin and tetanolysin. Tetanolysin damages the viable tissue around the infected wound, lowers the redox potential in the wound, and further facilitates the growth of anaerobic organisms. Tetanospasmin causes the clinical manifestations of tetanus.
Tetanospasmin is a single, 1315-amino-acid polypeptide that acts as a zinc-dependent peptidase. The molecule becomes
toxic after being cleaved at serine 458 by a bacterial protease into a heterodimer of a 100-kDa heavy chain and a 50-kDa light chain connected by a disulfide bridge. The heavy chain is further cleaved by pepsin into B and C fragments. The resulting toxin thus comprises the amino-terminal end of a heavy chain (fragment B) linked with a light chain (fragment A) by a disulfide bridge (fragment A-B) and a heavier carboxyl-terminal polypeptide (fragment C). Fragment C is responsible for attachment to the neuronal cell surface receptors and internalization of toxin, while fragment A-B produces the presynaptic inhibition of neurotransmitter release that results in clinical tetanus.
After entering the body, tetanospasmin spreads via lymphatics and blood vessels to enter the nervous system at the neuromuscular junction of the lower motor neurons. Although its greatest affinity is for inhibitory systems, a small amount of toxin may also enter sensory and autonomic neurons. The receptor to which toxin binds is thought to be membrane gangliosides, but this remains controversial. The toxin spreads through the central nervous system (
CNS) by retrograde axonal transport to the cell body and transsynaptically to other neurons, particularly the presynaptic inhibitory neurons. The proteins synaptotagmin, syntaxin, and synaptobrevin are involved in docking of synaptic vesicles to presynaptic membrane and release of the contents of synaptic vesicles into the synaptic clefts. Tetanospasmin cleaves peptide bonds of synaptobrevin and inhibits the release of neurotransmitters, predominantly glycine, in the spinal cord and
γ-aminobutyric acid (
GABA) in the brainstem. The loss of inhibition of
a-motor neurons and dysfunction of polysynaptic reflexes result in inhibition of antagonists, causing sustained, uninhibited contraction of muscles (tetany). Excitatory transmission is also disrupted, causing weakness of muscles.
Blood-borne spread of toxin occurs from the site of entry to the brain at the area postrema of the floor of the fourth ventricle, where the blood-brain barrier (
BBB) is nonexistent. This possibly explains the early manifestations of tetanus, such as trismus and nuchal rigidity.
The autonomic dysfunction in tetanus is caused by several mechanisms, which include the effect of tetanolysin on brainstem and autonomic interneurons, and a direct effect of the toxin on the myocardium and adrenal inhibition. The loss of glycine inhibition by tetanospasmin affects preganglionic sympathetic neurons in spinal cord and causes increased sympathetic activity and increased catecholamine levels. Tetanospasmin may interfere with the release of acetylcholine in peripheral somatic and autonomic nerves, resulting in a progressive interference with the inhibition of neuronal transmission. Also, tetanospasmin probably has an angiotensin-converting enzyme-like effect that contributes to hypertension; inhibition by captopril of the effect of tetanospasmin on synaptobrevin supports this view.
Clinical Features
The incubation period—the time interval between spore inoculation and symptom onset—may vary from 2 days to months (average 2 weeks). In severe forms, the incubation period is shorter, and the period of onset is <48 hours. Tetanus typically evolves as one of four clinical forms, generalized, localized, neonatal, or cephalic.
Generalized Tetanus
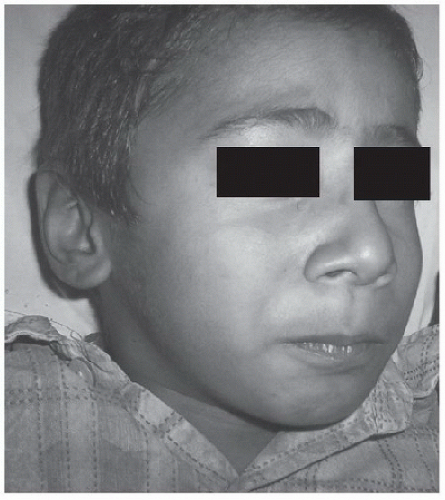
The most common form of tetanus is generalized tetanus, which manifests with classical trismus or “lockjaw”
(Fig. 94.1), followed by
risus sardonicus (a facial grimace that results from hypertonia of the orbicularis oris), generalized muscle rigidity, hyper-reflexia, dysphagia, opisthotonos,

and spasms. The body assumes an opisthotonic position that resembles decorticate posturing (without loss of consciousness) with flexion of the arms and extension of the legs. The muscle spasms are caused by a sudden burst of tonic contraction in muscles and are very painful. Pharyngeal spasms lead to severe dysphagia. If prolonged, spasms may lead to rhabdomyolysis (and its complications), laryngeal obstruction, acute respiratory failure, and cardiac arrest. Spasms are more prominent in the first 2 weeks, and their severity may increase during this period. Rigidity may last beyond the occurrence of spasms and autonomic disturbances, which usually occur some days after the spasms and reach a peak during the second week of the disease (
4). Sympathetic overactivity, associated with elevated plasma norepinephrine and epinephrine concentrations, causes fluctuating heart rate, peripheral pallor, labile hypertension, and fever with profound sweating. Fluctuations in blood pressure and heart rate appear to be related to changes in systemic vascular resistance rather than cardiac output or left-ventricular filling pressure. These may be accompanied by hypotension and cardiac arrhythmias (paroxysmal supraventricular tachycardia, runs of ventricular tachycardia, and ventricular or atrial premature beats). Parasympathetic involvement may manifest as excessive salivation and increased bronchial secretions as well as bradycardia and sudden cardiac arrest in severe tetanus. Recovery usually begins after 3 weeks and takes about 4 weeks. However, the clinical course is often unpredictable.
Localized Tetanus
Occasionally, muscle rigidity, often in association with muscle weakness, may remain localized at the site of spore inoculation. Symptoms may be mild, self-limited, and persistent (in partially immune hosts), or may progress to generalized tetanus.
Neonatal Tetanus
Neonatal tetanus is a generalized form of tetanus that develops through contamination of the umbilical stump in infants born to inadequately immunized mothers. The contamination
may be caused by the use of unsterile instruments to cut the cord or by unhygienic cord care practices (e.g., applying soil or cow dung dressing to the stump) that are prevalent in certain populations. The first signs are poor sucking and excessive crying, followed by variable degrees of trismus, risus sardonicus, and repeated generalized muscle spasms. Apnea may result from spasm of respiratory muscles. The baby cannot be nursed and, unless appropriate treatment is available, is at high risk for death. If the baby survives, spasms subside by the late second or early third week, and swallowing returns by the end of 4 weeks.
Cephalic Tetanus
Cephalic tetanus is an uncommon localized form of disease that is often associated with otitis media and head injuries and affects the cranial nerves. Facial paresis is usually present. A coexisting aerobic infection, often caused by S. aureus, may be present. Rarely, extraocular movements are affected, causing “ophthalmologic tetanus.”
Complications of Tetanus
Hypoxemia that leads to respiratory failure is a major cause of death. Cardiovascular consequences of autonomic instability, including cardiac arrhythmias and cardiomyopathy, sometimes occur and may be less amenable to secondary prevention.
Patients who are treated in the
ICU are particularly prone to respiratory complications related to the use of mechanical ventilation (pneumothorax, atelectasis, ventilator-associated pneumonia), nosocomial infection, urinary tract infection related to indwelling catheters, wound sepsis, and gastrointestinal hemorrhage. The most common bacteria-causing infections are those that are Gram negative and
S. aureus.
Rhabdomyolysis may occur in severe generalized tetanus and may lead to acute renal failure. If the serum creatine kinase level is high or myoglobin is detected in the urine, hydration with normal saline and urinary alkalinization with sodium bicarbonate should be considered. Phrenic and laryngeal neuropathies and other mononeuropathies can occur as a consequence of tetanus. Compression of the common peroneal nerve at the fibular head may produce foot drop.
Management
Tetanus at any age is a medical emergency and is best managed in a referral hospital. Availability of intensive care and critical care protocols has made a significant impact on survival of patients with tetanus (
7). Grading of tetanus severity on the basis of Ablett criteria
(Table 94.3) may help in selecting patients who might most benefit from intensive monitoring and care. Management goals and a proposed protocol for management of severe grades of tetanus appear in
Table 94.4 and include:
1. Neutralization of the unbound toxin
2. Removal of the source of toxin

3. Control of rigidity and muscle spasms
4. Control of autonomic dysfunction
5. Supportive care—airway and ventilation
Neutralization of the Unbound Toxin
Passive immunization to neutralize circulating (unbound) toxin by using antitoxin shortens the course and reduces the severity of tetanus. Human tetanus immunoglobulin (
HTIG) is the preparation of choice. It consists of immunoglobulin G and has a half-life of 25 days. The dose of
HTIG is 500
IU given
IM or
IV, although doses as high as 3000-6000
IU have been used. Where
HTIG is unavailable, equine anti-tetanus serum may be used after testing for hypersensitivity. Equine anti-tetanus serum has a higher risk of anaphylactic reactions and a short half-life of 2 days but is less expensive. The usual dose of anti-tetanus serum is 500-1000 U/kg; half of the dose is given
IM, and half
IV. Although
HTIG and anti-tetanus serum have effect only on the unbound toxin (which is present in serum samples of only 10% of cases), either one of them
should be administered as soon as possible in all cases, irrespective of the duration or severity of the disease. Whether antitoxin should also be infiltrated locally at the portal of entry is unclear and needs scrutiny.
Intrathecal Administration of Antitoxin. Antitoxin can be administered intrathecally with the assumption that high concentrations of antitoxin in
CSF and around the nerve roots will neutralize the toxin bound to neurons in
CNS and act on the inhibitory interneurons preventing the release of
GABA. Some studies have found it effective, whereas others have not. A randomized trial from Brazil found shorter duration of occurrence of spasm, hospital stay, and respiratory assistance in adult patients treated with 1000
IU of intrathecal
HTIG (
8). A meta-analysis of 12 randomized studies that included 484 patients in the intrathecal group and 458 in the
IM group concluded that intrathecal therapy was superior to
IM therapy (
9). The superiority of intrathecal therapy also emerged when the analysis was performed separately for adults and neonates and for high and low doses. Intrathecal anti-TIG (250
IU intrathecal, after removal of equal volume of
CSF) in addition to the standard treatment improved the outcome of neonatal tetanus in terms of mortality and hospital stay (
10).
 Tetanus is caused by infection with Clostridium tetani, which elaborates a toxin-designated tetanospasmin, which blocks the release of inhibitory neurotransmitters, and causes uninhibited sustained muscle contraction.
Tetanus is caused by infection with Clostridium tetani, which elaborates a toxin-designated tetanospasmin, which blocks the release of inhibitory neurotransmitters, and causes uninhibited sustained muscle contraction. Trismus and risus sardonicus are the most common initial symptoms, progressing gradually to generalized rigidity and muscle spasms. Mental status is not affected. Cranial nerve palsies occur in cephalic tetanus. Signs of autonomic dysfunction, such as hypertension and tachyarrhythmias, occur 5-7 days after the onset of generalized spasms.
Trismus and risus sardonicus are the most common initial symptoms, progressing gradually to generalized rigidity and muscle spasms. Mental status is not affected. Cranial nerve palsies occur in cephalic tetanus. Signs of autonomic dysfunction, such as hypertension and tachyarrhythmias, occur 5-7 days after the onset of generalized spasms. Treatment in the ICU is focused on neutralization of circulating toxin with human tetanus immunoglobulin (500 IU); early intubation/tracheostomy if frequent generalized, pharyngeal or laryngeal spasms occur; penicillin or metronidazole administration; control of muscle spasms with IV benzodiazepines or with neuromuscular blockade in severe cases; and control of hypertension and tachycardia with morphine and either propranolol or labetalol.
Treatment in the ICU is focused on neutralization of circulating toxin with human tetanus immunoglobulin (500 IU); early intubation/tracheostomy if frequent generalized, pharyngeal or laryngeal spasms occur; penicillin or metronidazole administration; control of muscle spasms with IV benzodiazepines or with neuromuscular blockade in severe cases; and control of hypertension and tachycardia with morphine and either propranolol or labetalol. Diphtheria is a severe, widespread infectious disease that has the potential to reach epidemic proportions (as seen in Eastern Europe and the former Soviet Union in the 1990s cases and 2500 deaths worldwide in 2011).
Diphtheria is a severe, widespread infectious disease that has the potential to reach epidemic proportions (as seen in Eastern Europe and the former Soviet Union in the 1990s cases and 2500 deaths worldwide in 2011). Most infections are localized at a mucocutaneous site and can result in severe disease if produced by a toxigenic strain of C. diphtheriae, manifested by tonsillopharyngitis (90% cases) and pseudomembrane formation.
Most infections are localized at a mucocutaneous site and can result in severe disease if produced by a toxigenic strain of C. diphtheriae, manifested by tonsillopharyngitis (90% cases) and pseudomembrane formation. The diagnosis is confirmed by the isolation of C. diphtheriae from a clinical specimen or a fourfold or greater rise in serum antibody titers.
The diagnosis is confirmed by the isolation of C. diphtheriae from a clinical specimen or a fourfold or greater rise in serum antibody titers. Management consists of immediate administration of diphtheria antitoxin and antibiotics (penicillin or erythromycin), airway management, monitoring of cardiac function (heart rate, blood pressure, and electrocardiogram), and room isolation.
Management consists of immediate administration of diphtheria antitoxin and antibiotics (penicillin or erythromycin), airway management, monitoring of cardiac function (heart rate, blood pressure, and electrocardiogram), and room isolation. The airway should be secured early, as rapid progression of the membrane may preclude a later opportunity.
The airway should be secured early, as rapid progression of the membrane may preclude a later opportunity. All survivors must be immunized. All close contacts should be cultured, observed daily for signs of diphtheria for 7 days, and given antibiotic regardless of immunization status (oral erythromycin for 7-10 days or one dose of intramuscular benzathine penicillin).
All survivors must be immunized. All close contacts should be cultured, observed daily for signs of diphtheria for 7 days, and given antibiotic regardless of immunization status (oral erythromycin for 7-10 days or one dose of intramuscular benzathine penicillin). Immunized household contacts should receive a booster dose of toxoid, and unimmunized household contacts should receive primary immunization.
Immunized household contacts should receive a booster dose of toxoid, and unimmunized household contacts should receive primary immunization. Neurologic symptoms start with autonomic changes and oculobulbar muscle weakness, followed by symmetric descending weakness of limbs. Sensory system and mentation are usually spared.
Neurologic symptoms start with autonomic changes and oculobulbar muscle weakness, followed by symmetric descending weakness of limbs. Sensory system and mentation are usually spared. Diagnosis is based on clinical symptoms to avoid delay in treatment. Detection of toxin in patient’s serum, stool, or suspected food confirms the diagnosis.
Diagnosis is based on clinical symptoms to avoid delay in treatment. Detection of toxin in patient’s serum, stool, or suspected food confirms the diagnosis. Human botulism immune globulin is reserved for infants. Older children should receive equine botulinum toxin.
Human botulism immune globulin is reserved for infants. Older children should receive equine botulinum toxin. Supportive treatment should include support of airway and respiratory status. Elective intubation should be considered.
Supportive treatment should include support of airway and respiratory status. Elective intubation should be considered. Toxic shock syndrome (TSS) is an acute, toxin-mediated, multisystem febrile illness mainly caused by Staphylococcus aureus and group A Streptococcal infections.
Toxic shock syndrome (TSS) is an acute, toxin-mediated, multisystem febrile illness mainly caused by Staphylococcus aureus and group A Streptococcal infections. TSS is characterized by an abrupt onset of high fever, vomiting, and erythematous rash with rapid deterioration to hypotension and variable degrees of multiorgan failure.
TSS is characterized by an abrupt onset of high fever, vomiting, and erythematous rash with rapid deterioration to hypotension and variable degrees of multiorgan failure. Staphylococcal TSS has two forms: menstrual (associated with tampon use) and nonmenstrual. TSST-1 is found in nearly all cases of menstrual TSS and in 50% of nonmenstrual TSS, while enterotoxins are found in the other 50% of nonmenstrual TSS.
Staphylococcal TSS has two forms: menstrual (associated with tampon use) and nonmenstrual. TSST-1 is found in nearly all cases of menstrual TSS and in 50% of nonmenstrual TSS, while enterotoxins are found in the other 50% of nonmenstrual TSS. Streptococcal TSS is characterized by early shock and multiorgan failure and often accompanies invasive infections (e.g., rapidly progressive necrotizing fasciitis), sometimes in previously healthy patients.
Streptococcal TSS is characterized by early shock and multiorgan failure and often accompanies invasive infections (e.g., rapidly progressive necrotizing fasciitis), sometimes in previously healthy patients. The goals of management are removal of the source of toxin, appropriate antibiotic therapy, surgical debridement of necrotic tissues, neutralization of toxin with intravenous immunoglobulin therapy (1 g/kg for 2 days), and aggressive supportive therapy for shock (fluids and vasopressors) and multiorgan failure. Clindamycin is recommended for streptococcal TSS and in combination with a β-lactamaseresistant antistaphylococcal antibiotic (cloxacillin, oxacillin, or nafcillin) for staphylococcal TSS.
The goals of management are removal of the source of toxin, appropriate antibiotic therapy, surgical debridement of necrotic tissues, neutralization of toxin with intravenous immunoglobulin therapy (1 g/kg for 2 days), and aggressive supportive therapy for shock (fluids and vasopressors) and multiorgan failure. Clindamycin is recommended for streptococcal TSS and in combination with a β-lactamaseresistant antistaphylococcal antibiotic (cloxacillin, oxacillin, or nafcillin) for staphylococcal TSS. caused by the action of tetanospasmin (commonly called tetanus toxin), a potent neurotoxin elaborated by the organism Clostridium tetani (C. tetani).
caused by the action of tetanospasmin (commonly called tetanus toxin), a potent neurotoxin elaborated by the organism Clostridium tetani (C. tetani). and spasms. The body assumes an opisthotonic position that resembles decorticate posturing (without loss of consciousness) with flexion of the arms and extension of the legs. The muscle spasms are caused by a sudden burst of tonic contraction in muscles and are very painful. Pharyngeal spasms lead to severe dysphagia. If prolonged, spasms may lead to rhabdomyolysis (and its complications), laryngeal obstruction, acute respiratory failure, and cardiac arrest. Spasms are more prominent in the first 2 weeks, and their severity may increase during this period. Rigidity may last beyond the occurrence of spasms and autonomic disturbances, which usually occur some days after the spasms and reach a peak during the second week of the disease (4). Sympathetic overactivity, associated with elevated plasma norepinephrine and epinephrine concentrations, causes fluctuating heart rate, peripheral pallor, labile hypertension, and fever with profound sweating. Fluctuations in blood pressure and heart rate appear to be related to changes in systemic vascular resistance rather than cardiac output or left-ventricular filling pressure. These may be accompanied by hypotension and cardiac arrhythmias (paroxysmal supraventricular tachycardia, runs of ventricular tachycardia, and ventricular or atrial premature beats). Parasympathetic involvement may manifest as excessive salivation and increased bronchial secretions as well as bradycardia and sudden cardiac arrest in severe tetanus. Recovery usually begins after 3 weeks and takes about 4 weeks. However, the clinical course is often unpredictable.
and spasms. The body assumes an opisthotonic position that resembles decorticate posturing (without loss of consciousness) with flexion of the arms and extension of the legs. The muscle spasms are caused by a sudden burst of tonic contraction in muscles and are very painful. Pharyngeal spasms lead to severe dysphagia. If prolonged, spasms may lead to rhabdomyolysis (and its complications), laryngeal obstruction, acute respiratory failure, and cardiac arrest. Spasms are more prominent in the first 2 weeks, and their severity may increase during this period. Rigidity may last beyond the occurrence of spasms and autonomic disturbances, which usually occur some days after the spasms and reach a peak during the second week of the disease (4). Sympathetic overactivity, associated with elevated plasma norepinephrine and epinephrine concentrations, causes fluctuating heart rate, peripheral pallor, labile hypertension, and fever with profound sweating. Fluctuations in blood pressure and heart rate appear to be related to changes in systemic vascular resistance rather than cardiac output or left-ventricular filling pressure. These may be accompanied by hypotension and cardiac arrhythmias (paroxysmal supraventricular tachycardia, runs of ventricular tachycardia, and ventricular or atrial premature beats). Parasympathetic involvement may manifest as excessive salivation and increased bronchial secretions as well as bradycardia and sudden cardiac arrest in severe tetanus. Recovery usually begins after 3 weeks and takes about 4 weeks. However, the clinical course is often unpredictable. wounds) supports the diagnosis. In patients with trismus or neck stiffness, diagnosis may be difficult in the early phase of disease in the absence of clear evidence of injury. A bedside “spatula test” developed by Apte and Karnad (94% sensitivity and 100% specificity) can be helpful. On insertion of a spatula (or tongue blade) that touches the posterior pharyngeal wall, if the patient gags and expels the spatula, the test is negative, whereas if the patient bites the spatula because of reflex masseter spasms, the test is positive for tetanus (5).
wounds) supports the diagnosis. In patients with trismus or neck stiffness, diagnosis may be difficult in the early phase of disease in the absence of clear evidence of injury. A bedside “spatula test” developed by Apte and Karnad (94% sensitivity and 100% specificity) can be helpful. On insertion of a spatula (or tongue blade) that touches the posterior pharyngeal wall, if the patient gags and expels the spatula, the test is negative, whereas if the patient bites the spatula because of reflex masseter spasms, the test is positive for tetanus (5).