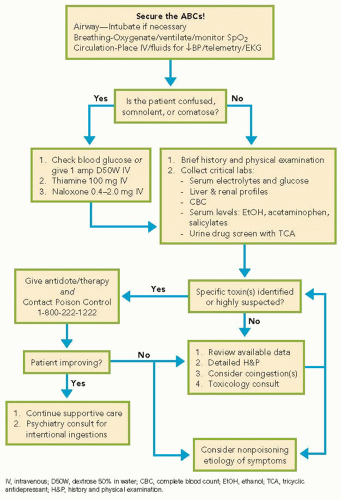
The Optimal Use of Laboratory Tests
Basic tests should include a comprehensive metabolic panel and complete blood cell count. Arterial blood should be analyzed by co-oximetry in the presence of respiratory distress, altered mental status, somnolence, coma, or cyanosis. Serum levels of ethanol, acetaminophen, and salicylate, and urine screen for common drugs of abuse and TCAs should be sent. Serum levels of digoxin, lithium, theophylline, phenytoin, and iron may be useful if the patient is known to take or have immediate access to these medicines. Do not delay treatment awaiting test results if an ingestion is suspected.
One should be cautious about the use and interpretation of toxicology screening tests. Qualitative urine or serum screening assays may reveal “exposure” to various classes of drugs, but cannot discern whether an exposure is responsible for the presentation. Many substances are not included on screens for drugs of abuse. Further, depending on the timing of the ingestion, coingestions, and the sensitivity of the test used, results may be falsely negative even in the presence of intoxication. Some tests evaluate for metabolites of a drug, and others may test for only some drugs in a particular drug class (e.g., some opiate screens are negative in the presence of methadone or fentanyl). Thus, toxicology screening tests can be used to support clinical suspicion, but management should be guided by a careful history and identification of a toxidrome, and therapy driven by clinical findings.
Clues to specific poisonings may be found by attention to the “three gaps”: the anion gap, the osmolal gap, and the oxygen saturation gap.
Anion gap elevations may indicate the ingestion of toxins such as ethylene glycol, methanol or salicylates. The formula for calculating the serum anion gap follows, and causes of an elevated or low anion gap are included in
Table 33.3.
The normal range is 7 to 13 mEq/L, but vary among laboratories.
Osmolal gap elevations may be present following toxic ingestions of alcohols. The serum osmolal gap is the difference between the measured and calculated serum osmolality. Thus, an elevated osmolal gap reflects the presence of an osmotically active substance in the blood that is not accounted for by routine calculation of osmolality. Formulas necessary for calculating the osmolal gap follow, and a list of causes of an elevated osmolal gap are included in
Table 33.4.
Osmcalculated = 2[Na+] + [urea]/2.8 + [glucose]/18 + [ethanol]/4.6 where [Na+] is in mmol/L and [urea], [glucose] and [ethanol] are in mg/dL.
Osmolal gap = Osmmeasured – Osmcalculated (normal < 10)
Oxygen saturation gap describes differences between oxyhemoglobin percentage as measured by pulse oximetry (SpO
2) or as estimated from arterial oxygen tension
(PaO
2) when compared with the oxyhemoglobin percentage (SaO
2) as measured by cooximetry. An oxygen saturation gap may indicate poisoning from carbon monoxide, cyanide or hydrogen sulfide, or the presence of an acquired hemoglobinopathy, as occurs with methemoglobinemia. If these toxins are suspected, arterial blood must be analyzed by a co-oximeter, which is capable of measuring the concentrations of oxyhemoglobin, deoxyhemoglobin, methemoglobin, and carboxyhemoglobin in the specimen.