KEY POINTS
Pneumothorax in critically ill patients is often missed with conventional chest radiography. Ultrasound is a more reliable means of detecting pneumothorax.
Pleural effusions can be detected by chest radiograph, chest CT and ultrasound. Ultrasound can be used for real time guidance of thoracentesis and chest tube placement.
Empyema is the presence of pus within the pleural space and should be treated with systemic antibiotics as well as insertion of a chest drain. Other relative indications for placement of a chest drain include: positive gram stain or culture of pleural fluid and/or pH <7.2.
Recurring pleural effusions (eg, malignancy) can be managed by placement of a tunneled drainage system or pleurodesis (chemical or surgical).
Pleurodesis is extremely painful and should always be preceded by aggressive anesthesia and analgesia.
Chest tubes placed for pneumothorax should be evaluated daily for air leak. Pleural drainage systems can usually be placed on water seal rather than suction. This may hasten the resolution of leak across the visceral pleura and thus hasten chest tube removal.
Chest tube removal can be considered when there is no air leak in the pleural drainage system (pneumothorax) and/or there is less than 100 to 300 mL of fluid drainage per day (effusion).
INDICATIONS FOR THORACOSTOMY
Thoracostomy tubes, alternatively called chest drains, are inserted to drain fluid or air from the pleural space and remain in place until the drainage is completed. The indications for thoracostomy placement differ based on the amount of air, characteristics of the fluid as well as the clinical and physiologic consequences of these pleural space collections.
A pneumothorax is defined as a collection of air within the pleural space. Often pneumothoraces can occur in otherwise healthy people (ie, primary spontaneous pneumothorax), but can also be postsurgical, iatrogenic, or related to trauma, including barotrauma from ventilator-induced lung injury. Secondary pneumothoraces occur in the setting of underlying lung disease. Symptoms of either a primary or secondary pneumothorax can include pleuritic chest pain or dyspnea; however, patients with secondary pneumothorax often have shortness of breath that is out of proportion to the size of the pneumothorax.31,32 Physical exam findings can be subtle, but can range from tachypnea and tachycardia to hypotension and cardiovascular collapse. Tracheal deviation away from the side of the pneumothorax and decreased breath sounds on the affected side as well as subcutaneous emphysema may be present.
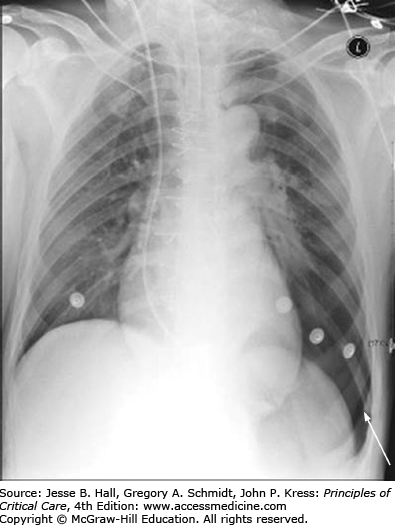
Imaging studies can be helpful in establishing a diagnosis. Chest computed tomography is the gold standard for diagnosis of pneumothorax. Indeed, nearly 40% of traumatic pneumothoraces are not clinically apparent.1 Chest roentography is a common method of identifying a pneumothorax once it is suspected clinically. Fully upright posteroanterior and lateral films are the most accurate roentographic method to identify a pneumothorax, although these are sometimes challenging to obtain, particularly in critically ill patients. A pneumothorax is identified by the presence of a dense white line with the absence of vascular markings lateral to it. At times, the patient’s positioning or lung pathology can cause collection of the air in either the anterior chest or along the costodiaphragmatic angle, creating a “deep sulcus” sign (Fig. 56-1).
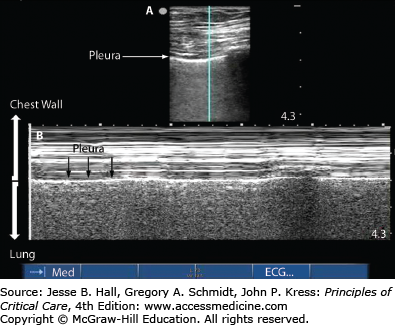
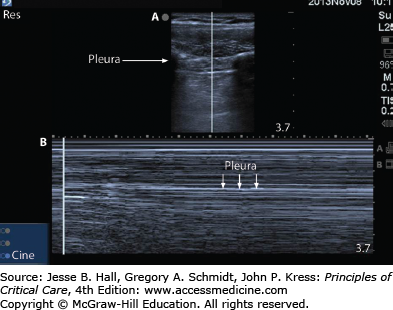
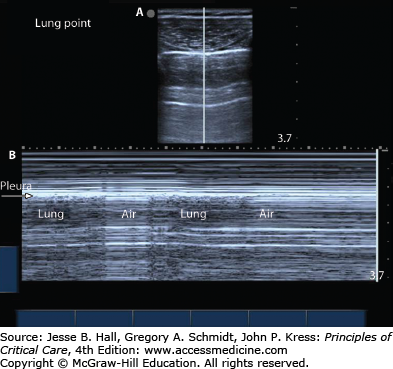
The use of ultrasound to image the lung and pleural space has become increasingly common. Ultrasound can be used by clinicians at the bedside to detect pneumothorax as soon as consistent signs/symptoms are identified. The interface between the aerated lung and the chest wall is readily visualized and often referred to as the pleural line. If this structure can be seen moving with respiratory variation, often referred to as lung sliding, then pneumothorax can be ruled out at that position.3,28 When the lung is imaged via ultrasound using the M-mode, or motion-mode, a normal lung demonstrates a seashore sign in which the lung appears grainy against the solid straight lines of the chest wall (Fig. 56-2). A pneumothorax appears as solid straight lines throughout the whole ultrasound field as is often referred to as the stratosphere or barcode sign (Fig. 56-3). Often, the transition between fully inflated lung and a pneumothorax can be identified: this is called the lung point (Fig. 56-4). It is characterized by normal sliding lung immediately adjacent to nonsliding lung. When M-mode is used to identify the lung point, the operator should visualize alternating seashore and stratosphere signs as the normal lung moves in and out of view. If the lung point can be found, it is highly specific for the presence of a pneumothorax.4 Furthermore, by scanning across the entire hemithorax, the lung point can be used to quantify the size of a pneumothorax.5 When compared to the gold standard of chest computed tomography, ultrasound is highly sensitive (86%-98%) and highly specific (97%-100%).5,6 Pneumothoraces that are missed on ultrasonography are small and typically do not require drainage.7 Ultrasound has been found repeatedly to be more sensitive and specific than chest roentography.
FIGURE 56-2
Normal lung imaging: M-mode seashore sign. Figure 56-2A Normal static appearance of the chest wall, pleura, and lung as visualized by ultrasound. [Vertical] line represents the plane through which structures are plotted over time for M-mode as shown in Fig. 56-2B. Figure 56-2B Seashore sign: Normal appearance of the chest wall, pleura and lung over a 5-second interval as visualized by ultrasound using M-mode.
FIGURE 56-3
Typical static appearance of a pneumothorax as visualized by ultrasound (see Fig. 56-3A). [Vertical] line represents the plane through which structures are plotted over time for M-mode as shown in Figure 56-3B. Stratosphere sign: Appearance of pneumothorax over a 10-second interval as visualized by ultrasound using M-mode. Note the repeating gray lines throughout the entire ultrasound field which suggest the presence of a pneumothorax.
FIGURE 56-4
Typical static appearance of a pneumothorax as visualized by ultrasound (see Fig. 56-4A). [Vertical] line represents the plane through which structures are plotted over time for M-mode as shown in Figure 56-4B. Lung point: Appearance of pneumothorax over a 10-second interval as visualized by ultrasound using M-mode. Note the different ultrasound appearance during a respiratory cycle as the probe visualizes the air filled pleura alternating with the air filled lung.
Pneumothorax size, symptoms of breathlessness or the presence of underlying lung disease is often used as criteria to determine whether to drain pleural air, place a chest drain or observe for spontaneous resolution.2 Importantly, if a patient is unstable and is demonstrating signs and symptoms that are suggestive of a pneumothorax, intervention must not be delayed for traditional imaging studies. If the patient is asymptomatic with a primary pneumothorax <2 cm (measured from either the chest wall at the level of the hilum to the pleural line or the lung apex to the cupola) then observation is an acceptable management strategy with follow up as an outpatient. Most experts recommend aspiration of pleural air in a breathless patient with a primary pneumothorax that is >2 cm or in an asymptomatic patient with a secondary pneumothorax that is 1 to 2 cm. If air aspiration improves symptoms and the resulting pneumothorax is <2 cm then a patient with a spontaneous pneumothorax can be followed as an outpatient. In a patient with a secondary pneumothorax air aspiration resulting in improved breathlessness and a reduction in the size of the pneumothorax to <1 cm is deemed a success: these patients should be observed for up to 24 hours to ensure stability given their underlying lung disease. The presence of breathlessness or a pneumothorax >2 cm in the setting of a secondary pneumothorax or an inadequate response to air aspiration in a primary pneumothorax should prompt the physician to insert a chest drain to fully evacuate pleural air.2
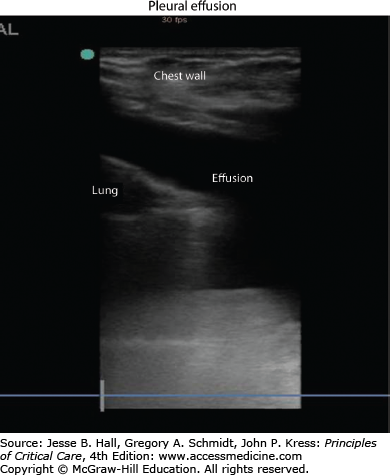
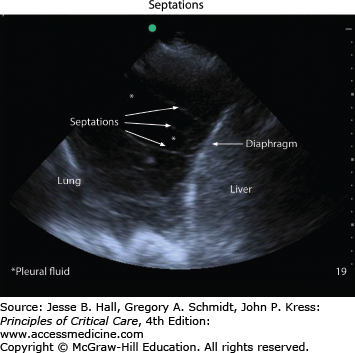
Chest imaging should be performed when a patient’s clinical presentation or exam is suggestive of pleural effusion. A posteroanterior chest x-ray is often helpful in confirming the presence of pleural effusion and a lateral x-ray can often have additional value.30 Chest computed tomography can identify smaller effusions and is better able to delineate the characteristics of a complicated effusion. Increasingly, ultrasonography is used to localize pleural fluid, quantify its size, and guide sampling of the fluid. The use of ultrasound in real-time procedural guidance helps to increase the success rate and decrease the complication rate of thoracentesis.8-10 Effusions typically appear as an anechoic space (Fig. 56-5), although echogenicity within the fluid can be a sign of a complicated process such as empyema or hemothorax.3 Septations, adhesions, and loculations can also be identified by ultrasound (Fig. 56-6). The presence of a pleural effusion should always prompt the clinician to consider the etiology for the fluid accumulation. Most experts agree that pleural effusions should be diagnostically sampled in the setting of suspected infection.33 Fluid analysis reveals whether the effusion is a transudate, an exudate or an empyema and thus helps guide the decision about performing a tube thoracostomy. Transudative pleural effusions, with rare exception,11 do not require tube thoracostomy. More commonly, chest tube insertion is required for exudative effusions: this includes empyema, hemothorax, and malignant pleural effusions.
Empyema is the presence of pus within the pleural space and should be treated with systemic antibiotics as well as insertion of a chest drain. Other indications for placement of a chest drain for pleural space infection include: positive gram stain or culture of pleural fluid and/or pH <7.2. Once the drain is in place, clinical improvement and radiographic confirmation of effusion drainage are used to determine when the drain can be removed. Incomplete pleural space drainage in the setting of persistent signs of infection should lead to consideration of surgical exploration of the pleural space. In patients who are not suitable for surgery, intrapleural fibrinolysis can be considered. However, several trials have shown that intrapleural fibrinolysis alone does not reduce mortality or the incidence of surgery for pleural infections so this option should be reserved for patients unable to tolerate surgery.12-14 One study showed that the combined use of tissue plasminogen activator (t-PA) and DNAse was associated with a reduction in the need for surgical referral at three months and the duration of hospitalization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree