The Upper Extremity: Somatic Block
Joseph M. Neal
Upper extremity regional anesthesia is the most common form of peripheral nerve block performed by anesthesiologists, even when intravenous (IV) regional anesthesia techniques are excluded. Fellowship-trained regional anesthesiologists also perform more upper than lower extremity regional anesthesia (1). This chapter provides a comprehensive review of brachial plexus regional anesthesia, including its affect on patient outcome, the anatomy and pharmacology of the brachial plexus, complications specific to these techniques, and practical advice on performing regional anesthesia at the various approaches to the brachial plexus.
Hypertension and Bradycardia
Nonanesthesiologists may surmise that regional anesthesia of the brachial plexus is safer for patients than is general anesthesia. No evidence supports this belief. Indeed, the prudent anesthesiologist recognizes that in certain patient subsets, regional anesthesia may be riskier than general anesthesia despite an intuitive sense to the contrary. For example, upper extremity regional anesthesia may be presumed safer than general anesthesia in patients with severe pulmonary disease, when in fact above-the-clavicle approaches place these patients at risk for further pulmonary compromise from transient hemidiaphragmatic paresis. The absence of evidence that upper extremity block reduces major morbidity should not be surprising, since upper extremity surgery itself does not adversely impact cardiac or pulmonary pathophysiology. However, upper extremity regional anesthesia can positively impact lesser outcomes during the first 24 hours after surgery. Randomized clinical trials have consistently shown that the use of a regional anesthetic technique for shoulder (2), hand, or wrist (3,4) surgery improves analgesia; reduces opioid requirement, with consequent reduction of opioid-related side effects; lessens time to discharge; and lessens the need for hospital admission as compared to fast-track general anesthesia techniques. Furthermore, benefits such as superior analgesia and improved sleep can be prolonged if regional analgesia is extended into the postoperative period with the use of a continuous perineural catheter (5,6). Limited evidence suggests that continuous brachial plexus analgesia improves hard outcomes, such as better rehabilitation or earlier return to work (7). Preliminary studies suggest that continuous perineural techniques can aid early hospital discharge and facilitate rehabilitation on an outpatient basis, provide superior analgesia, and reduced opioid-related side effects after major procedures such as total shoulder arthroplasty (8,9). Importantly, the use of continuous perineural techniques does not appear to increase the risk of major neurologic or infectious complications, as compared to single-injection techniques. (10,11).
Brachial Plexus Anatomy
The Brachial Plexus
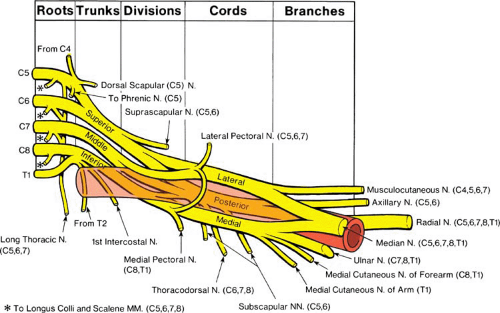
Brachial plexus anatomy can be daunting to skilled anatomists, and is even more so to the occasional practitioner of regional anesthesia. The brachial plexus forms from the ventral primary rami of the fifth through eighth cervical (C5–C8) and the first thoracic (T1) nerves. The C4 and T2 nerve roots may also contribute to the brachial plexus. From these separate nerve roots, the brachial plexus undergoes a complex series of convergences and divergences (Fig. 13-1). As the nerve roots traverse the space between the anterior and middle scalene muscles, they converge into three vertically arranged trunks (upper, middle, and lower), which shortly thereafter diverge into anterior and posterior divisions as they cross over the lateral border of the first rib. From this point forward, the posterior division innervates the posterior arm, primarily as the radial and axillary nerves. As the divisions proceed distally, they wrap around the second part of the axillary artery and form three distinct cords. The posterior cord is simply a continuation of the posterior division and lies posterior to the artery. The anterior division splits into the medial and lateral cords, which are defined by their relationship to the axillary artery. Both of these cords usually contain portions of what will eventually become the median nerve, while the musculocutaneous nerve is derived from the lateral cord and the ulnar nerve is derived from the medial cord. By the time the neurovascular bundle reaches the axilla, the four terminal nerves remain separate and distinct for the remainder of their distal travel, with the musculocutaneous nerve typically coursing within the belly or fascia of the coracobrachialis muscle (12).
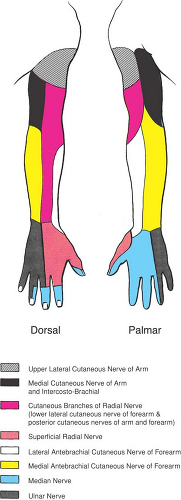
Structurally, the brachial plexus exhibits marked variation in its configuration; at least 29 known variations exist (13), and two–thirds of humans will have a different brachial plexus configuration on their right as compared to their left side (14). This variation in anatomic configuration is ultimately mirrored in upper extremity cutaneous sensory innervation. Schematic depictions of the arm’s sensory innervation seldom represent clinical reality (Fig. 13-2). Innervation of the arm is characterized by significant overlap of cutaneous distribution, especially in the volar forearm and hand, where the sensory fields blend and thus impair the anesthesiologist’s ability to assess which terminal nerves are adequately anesthetized or may require anesthetic
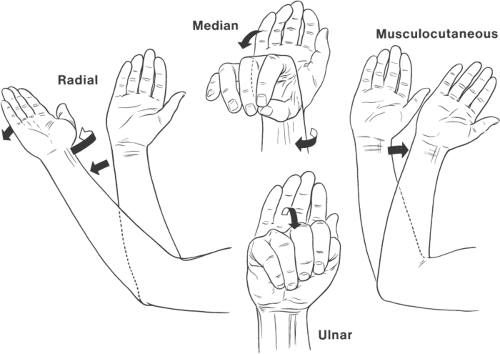
supplementation. A frequently used technique for assessing upper extremity anesthetic distribution isolates sensory or motor characteristics specific to each terminal nerve, rather than attempting to use and then potentially misinterpret traditional sensory or motor tests such as loss of pin-prick or grip strength. The practitioner can thereby more accurately link inadequate block to a specific nerve. This assessment technique—Push—Pull—Pinch—Pinch—isolates the four terminal nerves in the following manner. Inability to push by extending the forearm against resistance confirms anesthesia of the radial nerve. Loss of the ability to pull the forearm by flexing it against resistance signifies anesthesia of the musculocutaneous nerve. Inability to recognize pinches at the palmar bases of the index finger or the little finger indicates anesthesia of the median nerve and ulnar nerve, respectively (15).
supplementation. A frequently used technique for assessing upper extremity anesthetic distribution isolates sensory or motor characteristics specific to each terminal nerve, rather than attempting to use and then potentially misinterpret traditional sensory or motor tests such as loss of pin-prick or grip strength. The practitioner can thereby more accurately link inadequate block to a specific nerve. This assessment technique—Push—Pull—Pinch—Pinch—isolates the four terminal nerves in the following manner. Inability to push by extending the forearm against resistance confirms anesthesia of the radial nerve. Loss of the ability to pull the forearm by flexing it against resistance signifies anesthesia of the musculocutaneous nerve. Inability to recognize pinches at the palmar bases of the index finger or the little finger indicates anesthesia of the median nerve and ulnar nerve, respectively (15).
Basic understanding of upper extremity motor innervation is essential for correctly interpreting motor responses to peripheral nerve electrical stimulation. Shoulder elevation and/or biceps contraction signifies stimulation of the upper trunk and correct needle placement during the interscalene approach, whereas posterior shoulder movement is unacceptable because it indicates dorsal scapular nerve stimulation and needle placement that is too posterior. Triceps movement indicates radial nerve stimulation, but is a less desirable end-point than extension of the wrist or fingers during axillary block (16). Musculocutaneous nerve stimulation results in forearm flexion. Median nerve stimulation causes forearm pronation, wrist flexion, and thumb/index finger opposition. Ulnar nerve stimulation is identified by ulnar deviation of the wrist, thumb adduction, and little finger flexion (Fig. 13-3).
Important Brachial Plexus Branches and Non-brachial Plexus Nerves
Knowledge of certain important branches of the brachial plexus, together with select nerves that are not part of the brachial plexus, is crucial for intelligently providing upper extremity anesthesia (Fig. 13-1). The supraclavicular nerve (C3–C4) innervates the “cape of the shoulder.” Although it is frequently blocked from cephalad local anesthetic spread during the interscalene approach, adequate anesthesia is often absent after the supraclavicular brachial plexus approach. The suprascapular nerve (C5–C6) branches from the upper trunk. Depending on how proximally this branching occurs in relationship to where the block needle is placed, the suprascapular nerve may require a separate anesthetizing procedure for surgery involving the shoulder joint. The intercostobrachial nerve (T2) is not part of the brachial plexus and therefore requires separate anesthesia when surgery involves the upper posterior medial arm. The medial antebrachial cutaneous nerve innervates the medial volar forearm and may remain unanesthetized when an axillary or midhumeral block is placed distal to where it branches from the medial cord.
Important Anatomic Relationships
The brachial plexus maintains relatively consistent anatomic relationships with surrounding structures that aid in the provision of upper extremity regional anesthesia (Fig. 13-4). As the
cervical nerve roots exit their respective intervertebral foramina, they invaginate the prevertebral fascia, which purportedly forms the brachial plexus sheath, the clinical significance of which is controversial (14,17,18,19,20,21). Early descriptions of the brachial plexus sheath described it as a tubular structure containing the various components of the brachial plexus—from the roots extending to beyond the axilla (21). Newer imaging methodologies place this concept into question and instead describe the brachial plexus as being contained within a tissue plane defined by the rigid structures of the chest wall, humerus, pectoral fascia, and scapula (17). This tissue plane, rather than a sheath, provides the structural conduit that facilitates tracking of injected local anesthetic solutions along the plexus. Proximally, the convergence and divergence of the trunks, roots, and divisions are mirrored by their accompanying connective tissues (22), which likely facilitates the even spread of anesthesia associated with blocks placed at these approaches (17). At the level of the first rib, cryomicrotome evidence suggests that the structural integrity of these connective tissues becomes less robust (14) (Fig. 13-5). Farther distally, as the terminal nerves and their connective tissue coverings become separate and distinct, the tubular integrity of the sheath and/or the rigid anatomy of the tissue plane become less apparent and less clinically relevant (18,19,20). The distal incongruity of tissue planes and connective tissues can be correlated with clinical evidence that multiple-injection techniques at these levels are generally more successful than single-injection techniques (14).
cervical nerve roots exit their respective intervertebral foramina, they invaginate the prevertebral fascia, which purportedly forms the brachial plexus sheath, the clinical significance of which is controversial (14,17,18,19,20,21). Early descriptions of the brachial plexus sheath described it as a tubular structure containing the various components of the brachial plexus—from the roots extending to beyond the axilla (21). Newer imaging methodologies place this concept into question and instead describe the brachial plexus as being contained within a tissue plane defined by the rigid structures of the chest wall, humerus, pectoral fascia, and scapula (17). This tissue plane, rather than a sheath, provides the structural conduit that facilitates tracking of injected local anesthetic solutions along the plexus. Proximally, the convergence and divergence of the trunks, roots, and divisions are mirrored by their accompanying connective tissues (22), which likely facilitates the even spread of anesthesia associated with blocks placed at these approaches (17). At the level of the first rib, cryomicrotome evidence suggests that the structural integrity of these connective tissues becomes less robust (14) (Fig. 13-5). Farther distally, as the terminal nerves and their connective tissue coverings become separate and distinct, the tubular integrity of the sheath and/or the rigid anatomy of the tissue plane become less apparent and less clinically relevant (18,19,20). The distal incongruity of tissue planes and connective tissues can be correlated with clinical evidence that multiple-injection techniques at these levels are generally more successful than single-injection techniques (14).
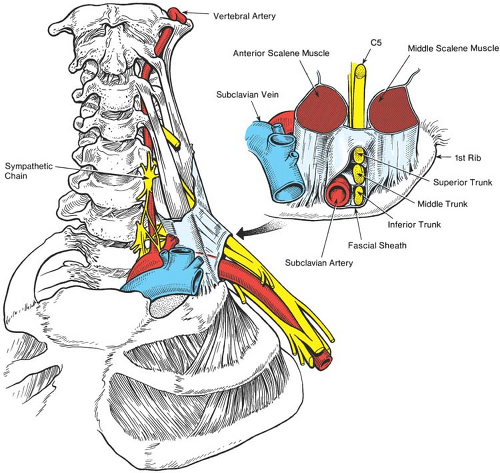
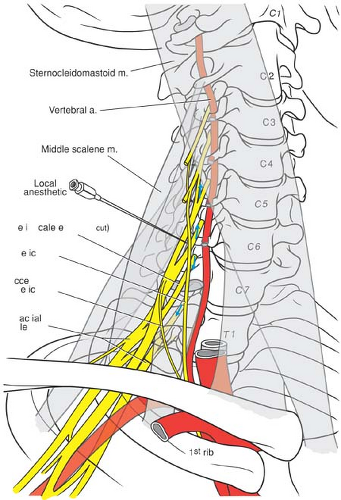
Muscular and vascular structures help to further guide and refine upper extremity block. The brachial plexus typically passes between the anterior and middle scalene muscles as it traverses from the neck to the arm in a lateral, 45-degree anterior, and caudad trajectory. Portions of the nerve roots occasionally pass directly through the anterior scalene muscle. The relationship of brachial plexus to scalene muscles defines the interscalene groove and provides surface landmarks used for the interscalene and some supraclavicular approaches (Fig. 13-4). Key vascular relationships include the vertebral arteries, which lie anterior to the nerve roots as they exit the intervertebral foramina, a position that makes them susceptible to intravascular injection from needles placed too medially and deeply (Fig. 13-6). The brachial plexus consistently resides posterior, lateral, and cephalad to the subclavian artery at the level of the first rib (Fig. 13-5). This relationship provides valuable information if blood is aspirated during the supraclavicular approach. The second part of the axillary artery defines the anatomic positioning of the three cords. More distally at the axilla, the four terminal nerves maintain a variably predictable relationship to the axillary artery (14) (Fig. 13-1). The dome of the pleura resides anterior and medial to the lower trunk (Fig. 13-5).
Brachial Plexus Pharmacology
Local Anesthetics
Local Anesthetic Selection
Selection of local anesthetic for upper extremity regional anesthesia is predicated primarily on the desired duration of surgical anesthesia and/or postoperative analgesia. Despite some favorable preliminary reports, no extended-release local anesthetic preparation is commercially available (23). Extended block duration may be unwise for surgeries that are expected to generate
mild to moderate pain, because some patients are distressed by the numbness and heaviness that accompany long-acting local anesthetic blockade. The possibility of compartment syndrome should be determined in consultation with the surgeon, since even analgesic blocks can mask early signs of impaired circulation. Prolonged sensory and especially motor block demand that patients are provided with protective slings and counseled to avoid sources of heat, cold, or trauma that could injure their insensate arm Using proper precautions, patients with anesthetized arms and/or continuous perineural infusions can be discharged home with minimal risk of injury (5,6,24).
mild to moderate pain, because some patients are distressed by the numbness and heaviness that accompany long-acting local anesthetic blockade. The possibility of compartment syndrome should be determined in consultation with the surgeon, since even analgesic blocks can mask early signs of impaired circulation. Prolonged sensory and especially motor block demand that patients are provided with protective slings and counseled to avoid sources of heat, cold, or trauma that could injure their insensate arm Using proper precautions, patients with anesthetized arms and/or continuous perineural infusions can be discharged home with minimal risk of injury (5,6,24).
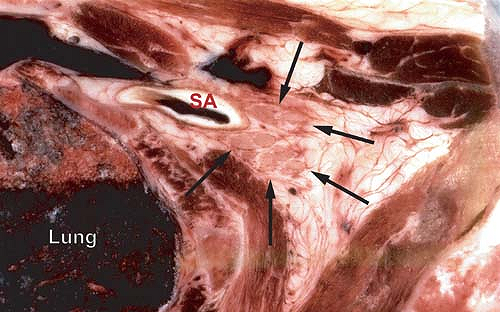 Figure 13-5. Cryomicrotome section at the base of the neck (top is anterior). Arrows denote the brachial plexus without an obvious surrounding sheath structure. The subclavian artery (SA) is posterior and lateral to the plexus. Note the proximity of the lung. Cryomicrotome by Quinn H. Hogan, MD. Used with permission of the American Society of Regional Anesthesia and Pain Medicine (14). |
Most upper extremity surgery is amenable to anesthesia using the intermediate-acting local anesthetics lidocaine and mepivacaine. When prolonged analgesia is desired, bupivacaine generally results in 8 to 14 additional hours of analgesia than mepivacaine, or 2 to 6 more hours as compared with ropivacaine. Plain bupivacaine applied to the brachial plexus for surgical anesthesia is approximately 33% more potent than plain ropivacaine (bupivacaine 0.5% = ropivacaine 0.75%) (25,26). Thus, the reduction in cardiotoxicity inherent to ropivacaine can be compromised if larger doses of ropivacaine are used in an attempt to mimic the characteristics of a bupivacaine
block. Levobupivacaine and racemic bupivacaine share similar anesthetic characteristics when applied to the brachial plexus (27).
block. Levobupivacaine and racemic bupivacaine share similar anesthetic characteristics when applied to the brachial plexus (27).
Local Anesthetic Dosing
For neuraxial local anesthetic blockade, total drug mass delivered to the target nerve is the most important factor in determining block effectiveness (28), yet its role is relatively inconsequential for brachial plexus blockade. Based on the studies of Vester-Andersen and colleagues (29,30,31,32), there is little evidence that brachial plexus block characteristics can be improved by manipulating local anesthetic dosing regimens. Specifically, block onset, quality, and duration are unaffected by increasing the volume, concentration, and/or total dose of local anesthetic. These data imply that many brachial plexus blocks are performed with more local anesthetic than necessary. Because systemic local anesthetic toxicity is related to the total dose delivered (33), and local anesthetic neurotoxicity in animal models is concentration-dependent (34), logic dictates that attempts to enhance brachial plexus block by using a high volume of concentrated local anesthetic not only fails to significantly improve block quality, but conceivably places the patient at increased risk should an unintended intravascular injection or nerve injury occur. Drug mass should be especially reduced in patients with altered local anesthetic metabolism, such as the elderly and those with congestive heart failure or liver disease (33).
Adjuvants
A myriad of adjuvants have been proposed to improve block quality in terms of faster onset, denser block, and/or longer analgesia. Clinical evidence suggests that only two adjuvants unequivocally accomplish these goals–epinephrine and clonidine—and that their most dramatic effects occur only in conjunction with the intermediate-acting local anesthetics lidocaine and mepivacaine. This phenomenon is explained by the inherent analgesic duration of long–acting local anesthetics being longer than the pharmacokinetic effects of the adjuvant.
Epinephrine intensifies block quality, prolongs block duration, decreases systemic uptake of local anesthetic (and thus the rapid rise of plasma levels that is most associated with systemic toxicity), and acts as a marker of intravascular injection. Epinephrine’s availability and low cost argue for its being the adjuvant of choice for brachial plexus blockade. When used in a 1:400,000 concentration (2.5 μg/mL), epinephrine extends local anesthetic blockade to almost the same degree as a 1:200,000 (5 μg/mL) concentration, but without concurrent tachycardia (35). Despite warnings to the contrary, there is no evidence that epinephrine significantly affects blood flow to end arteries, such as those in the fingers (36).
Clonidine prolongs anesthesia and analgesia of intermediate-acting local anesthetics by approximately 50%. When used in 0.5 μg/kg doses (<150 μg), clonidine does not cause significant hypotension, bradycardia, or sedation (37,38). In the United States, the use of clonidine is somewhat restricted by its expense relative to epinephrine and its inability to act as an intravascular marker. There is no benefit to adding clonidine to perineural infusions (39,40).
One other adjuvant—buprenorphine 0.3 mg—has been shown in a single study to increase the duration of mepivacaine upper extremity blocks. All other adjuvants, including opioids, neostigmine, hyaluronidase, tramadol, and calcium channel blockers (14), and most recently dexamethasone (41) and magnesium (42), have not been consistently shown to improve local anesthetic blockade of the brachial plexus and/or have unresolved toxicity issues.
The practice of alkalinizing local anesthetics to hasten block onset is beneficial in some neuraxial models, but not so for brachial plexus blocks. The addition of sodium bicarbonate (NaHCO3) to plain local anesthetic or to local anesthetic freshly admixed with epinephrine (43) does not result in significantly faster block onset at the brachial plexus; indeed, animal studies suggest that alkalinization of plain lidocaine reduces block quality and duration (44). The addition of NaHCO3 to local anesthetic premixed with epinephrine results in statistically, but perhaps not clinically, significant faster block onset. There is no evidence that alkalinizing local anesthetics improves other measures of block quality (14).
Complications Unique to Brachial Plexus Anesthesia
Neurologic and Infectious Complications
Neurologic and infectious complications associated with upper extremity regional anesthesia are exceedingly rare. Details of these complications are presented in Chapters 12 and 20.
Unintended Local Anesthetic Destinations
Intravascular Injection
As compared to epidural anesthesia, unintentional intravascular injection during peripheral nerve block is five times more likely to result in seizures (45). This observation is particularly important in brachial plexus anesthesia, because injection into the nearby vertebral, carotid, or subclavian arteries transports local anesthetic directly to the brain. Predictably, seizure is approximately five times more likely to be associated with the interscalene or supraclavicular approaches than with the axillary approach (46). Intraarterial injections are characterized by immediate seizure activity that resolves quickly if the injection is promptly stopped, which emphasizes the importance of using a 1-mL local anesthetic test dose. Subsequent fractionated 5-mL aliquots of local anesthetic (preferably with epinephrine as an intravascular marker) allow time to develop signs of local anesthetic systemic toxicity and/or epinephrine-induced tachycardia before systemic toxicity occurs from IV injection (47).
Neuraxial Anesthesia
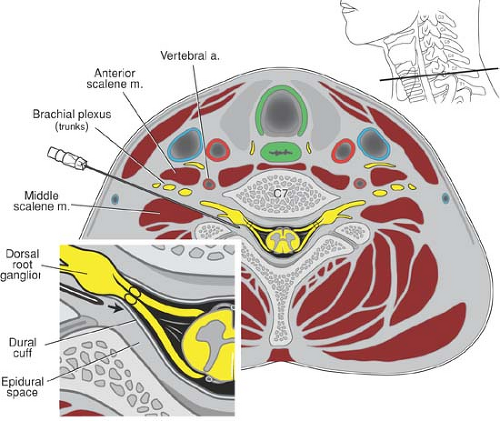
Unintended neuraxial anesthesia can result from a malpositioned needle during the interscalene approach, although the frequency of this complication may be decreased with technique modification (48,49). The distance from the skin overlying the interscalene groove to the intervertebral foramen can be as little as 25 mm (49), and as little as 35 mm to the neuraxis (50). A needle placed too deeply can thus enter the epidural, subdural, or subarachnoid space. Because some nerve root dural sleeves extend far laterally, even properly placed needles can gain access to cerebrospinal fluid and cause unexpected high spinal anesthesia (14) (Fig. 13-6). The use of shorter (25-mm) needles and fractionated dosing regimens may decrease the incidence of this rare complication, but no studies support these recommendations (51).
The presentation of unintended neuraxial anesthetic is determined by where the local anesthetic is injected. Subarachnoid injection results in rapid, bilateral anesthesia that extends from the cervical levels to the lower extremities and may result in immediate unconsciousness and/or apnea. Epidural anesthesia is delayed for 5 to 15 minutes and is more segmental and perhaps unilateral in presentation. In either case, bradycardia and hypotension are common signs (14). Similar to spinal anesthesia-induced hypotension and bradycardia, anesthetic blockade of the cardioaccelerator fibers and sympathetic vasomotor fibers require that resuscitation is rapid and aggressive, including the early use of epinephrine if hemodynamic perturbations fail to immediately respond to atropine and/or ephedrine. Needles can also be unintentionally placed through the intervertebral foramen and into the spinal cord (52).
Cervicothoracic Sympathectomy
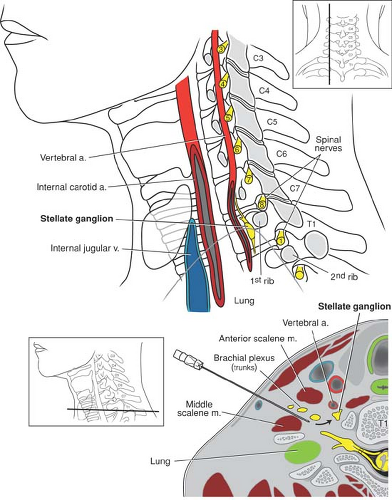
The cervicothoracic sympathetic trunk lies in close proximity to the lower brachial plexus (Fig. 13-7). It is therefore not
surprising that 20% to 90% of supraclavicular (53), 60% of cervical paravertebral (54), 7% of infraclavicular (55), and some interscalene blocks are associated with the development of ipsilateral Horner syndrome (miosis, ptosis, anhydrosis). This nuisance side effect resolves in concert with local anesthetic block resolution.
surprising that 20% to 90% of supraclavicular (53), 60% of cervical paravertebral (54), 7% of infraclavicular (55), and some interscalene blocks are associated with the development of ipsilateral Horner syndrome (miosis, ptosis, anhydrosis). This nuisance side effect resolves in concert with local anesthetic block resolution.
Recurrent Laryngeal Nerve Anesthesia
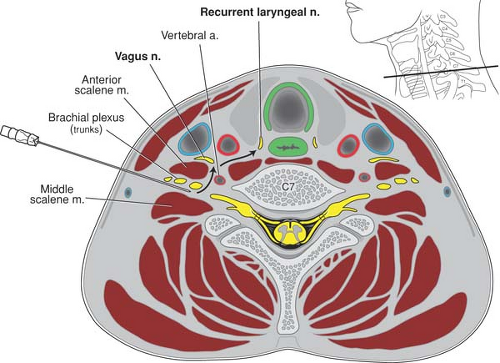
Local anesthetic injected into the neck during proximal approaches to the brachial plexus can also diffuse through tissue planes and affect the recurrent laryngeal and/or the vagus nerve (Fig. 13-8). When this occurs, hoarseness results. The reported incidence is higher with the interscalene approach (6% to 12%) (56) than it is with the supraclavicular approach (<2%) (53). This nuisance side effect will resolve along with dissipation of the local anesthetic block (51).
Hemidiaphragmatic Paresis
Ipsilateral hemidiaphragmatic paresis occurs when local anesthetic deposited around the brachial plexus unintentionally blocks the phrenic or accessory phrenic nerve. Although this can take place from direct local anesthetic contact with the phrenic nerve as it crosses the anterior scalene muscle, it most typically results from cephalad spread of local anesthetic to the C3-C5 nerve roots (57) (Fig. 13-9). The incidence and severity of hemidiaphragmatic paresis varies with the approach to the brachial plexus; the more cephalad the approach, the more likely the diaphragm is affected. Hemidiaphragmatic paresis is associated with 100% of interscalene blocks and can reduce spirometric measures of pulmonary function by 25% to 33% (57,58). The incidence of hemidiaphragmatic paresis is much lower with the supraclavicular approach (∼50%) and has no effect on pulmonary function in healthy volunteers (59). The sternocleidomastoid approach is associated with up to 60% incidence of hemidiaphragmatic paresis (60). The vertical infraclavicular approach has a 26% incidence of hemidiaphragmatic impairment with accompanying, but clinically unapparent, diminution in pulmonary function (61), whereas the more distal coracoid approach rarely affects diaphragmatic function (62).
Diaphragm impairment mirrors the onset and waning of local anesthetic effects. Because hemidiaphragmatic paresis with associated diminution in pulmonary function is both prevalent and unpredictable when using proximal approaches to the brachial plexus, these blocks are contraindicated in patients with contralateral diaphragm or phrenic nerve dysfunction, contralateral pneumonectomy, or in patients who may not withstand a 25% reduction in pulmonary function and/or those with a forced vital capacity of less than 1 L (63). Neither digital pressure proximal to the injection site and/or lowering injected volume to 20 mL (14,64), nor the infusion of motor-sparing local anesthetics such as bupivacaine or ropivacaine appreciably and predictably reduces the incidence of hemidiaphragmatic
paresis (65,66,67). Single-injection ropivacaine 0.5% has been associated with diaphragm impairment (68), whereas interscalene analgesic infusion of ropivacaine appears to result in a similar incidence of hemidiaphragmatic paresis to that seen with IV opioid analgesia (69). Although most studies suggest that manipulation of local anesthetic type, dose, concentration, or volume have insignificant effect on pulmonary function after interscalene block, one study has shown that reducing the volume and concentration of bupivacaine can minimize adverse respiratory effects (70).
paresis (65,66,67). Single-injection ropivacaine 0.5% has been associated with diaphragm impairment (68), whereas interscalene analgesic infusion of ropivacaine appears to result in a similar incidence of hemidiaphragmatic paresis to that seen with IV opioid analgesia (69). Although most studies suggest that manipulation of local anesthetic type, dose, concentration, or volume have insignificant effect on pulmonary function after interscalene block, one study has shown that reducing the volume and concentration of bupivacaine can minimize adverse respiratory effects (70).
Pneumothorax
Pneumothorax is an especially feared complication of the supraclavicular approach and is rarely possible from wayward needles placed using the interscalene or sternocleidomastoid approaches. Although the supraclavicular approach has been associated with a 0.6% to 6% incidence of pneumothorax, these data refer to the classic, but rarely used, Kulenkampff technique (71). The more contemporary
“plumb-bob” (72) and subclavian perivascular (21) techniques were designed in part to reduce the risk of pneumothorax. Although there are no sufficiently powered studies to confirm this impression, no pneumothoraces were reported in separate studies of 1,001 (73) and 2,020 (74) subclavian perivascular blocks. The more medially placed vertical infraclavicular approach theoretically carries a small risk of pneumothorax; risk should be exceedingly low using the more lateral coracoid approach (75,76,77). Since the pleura is easily identifiable using ultrasound, it is speculated that this technology might further reduce the incidence of pneumothorax.
“plumb-bob” (72) and subclavian perivascular (21) techniques were designed in part to reduce the risk of pneumothorax. Although there are no sufficiently powered studies to confirm this impression, no pneumothoraces were reported in separate studies of 1,001 (73) and 2,020 (74) subclavian perivascular blocks. The more medially placed vertical infraclavicular approach theoretically carries a small risk of pneumothorax; risk should be exceedingly low using the more lateral coracoid approach (75,76,77). Since the pleura is easily identifiable using ultrasound, it is speculated that this technology might further reduce the incidence of pneumothorax.
Symptoms of pneumothorax are frequently delayed 10 to 12 hours after block placement, particularly in the absence of mechanical ventilation. This delay has obvious implications for performing these blocks in outpatients. The most common presenting symptom of pneumothorax in these patients is pleuritic chest pain, not dyspnea (63).
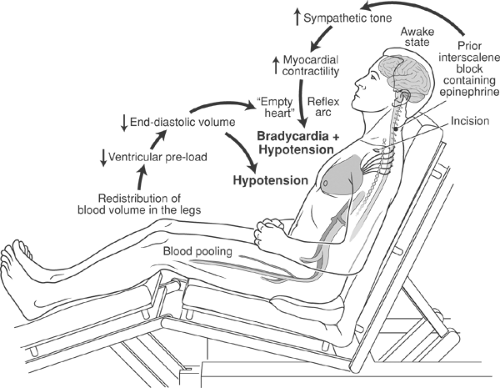
Hypotension Bradycardia
Interscalene block has been linked to the occurrence of hypotension and bradycardia in 13% to 24% of awake patients who undergo shoulder surgery in the beach chair position (78,79). The sudden onset of these hemodynamic alterations occurs about 60 minutes after block placement (78). The presumptive mechanism involves a hyperdynamic myocardium (associated with epinephrine used as block or irrigating solution additives, and/or from patient anxiety) that vigorously contracts against an empty ventricle (decreased preload from the beach chair position). These conditions combine to activate mechanoreceptors in the ventricular wall and reflexively cause bradycardia (Bezold-Jarish reflex) (Fig. 13-10). The frequency of this complication can be reduced by titrating metoprolol or similar β-blocker to a heart rate of less than 60 beats/min, but is unaffected by prophylactic glycopyrrolate (80).
Techniques to Optimize Block Success
Nerve Localization
The essence of regional anesthesia technique is safely placing the block needle sufficiently close to the target plexus or nerve
to ensure optimal local anesthetic effect. For upper extremity block, multiple options exist for localizing the target nerve, including paresthesia-seeking, peripheral nerve stimulation, ultrasound, and transarterial and perivascular techniques. Existing data suggest that no technique is clearly superior to another in terms of block success rates or safety (14,81,82). A growing body of knowledge and experience demonstrates that ultrasonic localization of target nerves is associated with highly successful and safe brachial plexus regional anesthesia (83). Yet, minimal evidence to date shows that ultrasound guidance improves success or safety as compared to other techniques. However, one must acknowledge that early investigators’ relative inexperience with ultrasound techniques, as compared to standard localization techniques, may potentially bias favorable outcomes toward the latter (84).
to ensure optimal local anesthetic effect. For upper extremity block, multiple options exist for localizing the target nerve, including paresthesia-seeking, peripheral nerve stimulation, ultrasound, and transarterial and perivascular techniques. Existing data suggest that no technique is clearly superior to another in terms of block success rates or safety (14,81,82). A growing body of knowledge and experience demonstrates that ultrasonic localization of target nerves is associated with highly successful and safe brachial plexus regional anesthesia (83). Yet, minimal evidence to date shows that ultrasound guidance improves success or safety as compared to other techniques. However, one must acknowledge that early investigators’ relative inexperience with ultrasound techniques, as compared to standard localization techniques, may potentially bias favorable outcomes toward the latter (84).
Of at least equal importance to localization technique is how many stimulations or injections should be carried out at each block approach. For above-the-clavicle blocks, a single localization end-point and subsequent injection is sufficient for successful block (14,81). However, as one proceeds distally along the course of the brachial plexus, multiple nerve localizations and injections clearly improve block success (82,85). Two injections are generally superior to a single injection for the infraclavicular (86) and transarterial axillary approaches (14), whereas three or more injections improve axillary block success when using a paresthesia, nerve stimulation, or perivascular technique (14,82).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree