(1)
Robert Wood Johnson Medical School, New Brunswick, NJ, USA
Keywords
SpleenSplenectomySplenorrhaphyIncisional herniaSmall bowel obstructionSepsisHemorrhageSplenic infarctionIntroduction
The spleen is one of the most commonly injured organs following blunt trauma. The injured spleen is a major source of morbidity and mortality and can be rapidly fatal if hemostasis is not achieved early. Up to one-third of patients with injured spleens proceed directly to the operating room for surgical exploration [1]. Patients who have had splenectomy are at risk for the usual morbidities of laparotomy such as incisional hernia and small bowel obstruction. Additionally, they are at increased risk for overwhelming sepsis syndromes due to asplenia. Nonoperative management also has risks including failure of nonoperative management, delayed hemorrhage, splenic infarction and abscess, and complications of angiography, if used. Given the common nature of the injury and the risks with all forms of management, the trauma surgeon must be well versed in the techniques required to care for this disease.
History of Care
Until recently, the spleen seemed a mystery, and it was considered nonessential for survival. The Talmud describes the spleen as a laughter-generating organ. Babylonian, Greek, and Roman texts incriminate the spleen as a cause of poor athletic performance [2]. Galen, Vesalius, and Malpighi all debated whether or not the spleen was necessary for survival [3]. There were accounts of asplenic patients, however, with no documented obvious adverse sequela suggesting lack of function of the organ.
During the early history of surgery for the injured spleen, splenectomy, though usually fatal, was considered as the only treatment for injury since its true function was unknown and death seemed more certain without operation. The spleen was considered frail and unable to heal. Early attempts at splenectomy for trauma described in the 1800s were quite often failures. Still, no consideration at that time was given to splenic salvage. It was incorrectly thought that the bone marrow and thyroid could substitute for the spleen’s perceived “blood producing quality.”
By the early twentieth century, however, splenectomy became more safe and hundreds of total splenectomies for trauma had been reported [4], many of them successful. Over the course of the twentieth century, the immunologic function of the spleen became better known and characterized. As early as 1952, data emerged that asplenic patients were at higher risk for overwhelming sepsis [5]. As this risk became better delineated, splenic salvage and conservative management of the spleen emerged as a management option [6].
Prior to the era of computed tomography (CT) scan, conservative management of splenic injury was splenorrhaphy or repair since diagnosis was usually made at laparotomy. With the advent and widespread use of CT scans, preoperative diagnosis became common. Concurrently, advances in catheter-based technologies allowed development of embolization. These technologies permitted true nonoperative, conservative management of the injured spleen. Splenectomy for trauma became significantly more uncommon.
The incidence of overwhelming postsplenectomy sepsis now appears lower than once perceived, perhaps due to advances in vaccine and prophylactic therapy. Further, compared to nonoperative management, the morbidity of laparotomy is high. One must weigh the benefits of definitive bleeding control and the risks of laparotomy to the potential benefit and risks of nonoperative management, including a defined failure rate. While in general, splenectomy for trauma might sometimes be a simple technical procedure; it is not one to be undertaken lightly due to the rare, but highly lethal, complication of overwhelming postsplenectomy sepsis. The remainder of the chapter is devoted to the issues that directly confront the trauma surgeon: how to diagnose and treat injuries of the spleen and care for complications of this treatment.
Techniques with Personal Tips
Techniques for Diagnosis
Approximately 30 % of patients with splenic injury are treated with primary operative exploration. Typically these patients present with hemodynamic instability and hemoperitoneum. While hemoperitoneum may be readily diagnosed in the trauma bay by focused assessment with sonography for trauma (FAST), the splenic injury as the source of the hemoperitoneum is typically diagnosed in the operating room (OR). Standard techniques of trauma laparotomy described earlier in this book should be followed, with packing of all four quadrants to obtain immediate hemostasis followed by controlled removal with inspection for injury and plans for prompt hemorrhage control as injuries are identified.
Many patients with splenic injury, however, are hemodynamically stable enough for diagnostic work-up to be performed after FAST in the trauma bay. In one review, ultrasound was 62–99 % sensitive for the detection of free intraperitoneal fluid. A large percentage of these patients will have splenic injury. However, up to 25 % of patients with splenic injury may not manifest hemoperitoneum [7]. A negative FAST in a hemodynamically stable patient who has significant mechanism concerning for solid organ injury should undergo further evaluation.
In most trauma centers, that diagnostic modality is multidetector CT imaging. CT provides very high sensitivity and specificity for diagnosing injury to the spleen. At the R Adams Cowley Shock Trauma Center, dual-phase imaging with both arterial phase and portal venous phase demonstrates the best diagnostic performance and can help guide management [8]. However, some patients may have discrete contraindications to intravenous (IV) contrast. While image quality may not be as good and detection of vascular lesions is limited, determination of significant injuries that require close follow-up or intervention can be made with a non-contrast-enhanced CT scan [9]. Once splenic injury is diagnosed, several important radiologic features should be evaluated as they impact treatment and outcomes: grade of injury, which should be based on the American Association for the Surgery of Trauma (AAST) grading system (Table 12.1) [10]; the presence or absence of active extravasation of IV contrast; the presence or absence of pseudoaneurysms; and the degree of hemoperitoneum. While CT does not detect all lesions, it detects lesions requiring treatment rather reliably, especially in high-grade injuries, and is useful for directing the initial care of the injured patient [11].
Table 12.1
AAST injury scale for the spleen.
Grade | Type | Description |
|---|---|---|
I | Hematoma | Subcapsular, <10 % surface area |
Laceration | Capsular tear, <1 cm parenchymal depth | |
II | Hematoma | Subcapsular, 10–50 % surface area; intraparenchymal, <5 cm in diameter |
Laceration | Capsular tear, 1–3 cm parenchymal depth that does not involve a trabecular vessel | |
III | Hematoma | Subcapsular, >50 % surface area or expanding; ruptured subcapsular or parenchymal hematoma; intraparenchymal hematoma ≥5 cm or expanding |
Laceration | >3 cm parenchymal depth or involving trabecular vessels | |
IV | Laceration | Laceration involving segmental or hilar vessels producing major devascularization (>25 % of spleen) |
V | Hematoma | Completely shattered spleen |
Laceration | Hilar vascular injury devascularizes spleen |
Techniques for Operative Treatment of Splenic Trauma
For the patient who is taken immediately to the operating room with hemodynamic instability, hemorrhage control must be quick. If at laparotomy, the spleen is found to be the cause of hemorrhage, splenectomy is the wisest choice in this patient. Our technique for emergent splenectomy is described:
While standing on the right side of the patient, the surgeon uses his/her right hand to rapidly mobilize the spleen by bluntly dividing the splenorenal and splenophrenic ligaments (Fig. 12.1a, b). The spleen is mobilized medially into the abdominal incision. It is an error to operate on the spleen in the left upper quadrant, as exposure is inadequate. The spleen can be elevated on some lap pads (Fig. 12.1c). If needed, a left medial visceral rotation or “Mattox maneuver” can be rapidly completed almost entirely with blunt dissection if other organs are injured in proximity to the spleen or retroperitoneal hemorrhage is identified in addition to splenic hemorrhage. We generally prefer to approach the spleen from its posterior aspect as it allows the best visualization of the pancreatic tail (Fig. 12.1d).The splenic vascular anatomy is variable. Sometimes, the splenic artery and vein extend onto the hilum. If so, they can be ligated flush with the spleen. Other times, they branch proximal to the hilum. If so, each branch should be ligated separately. The short gastric vessels are then ligated and the spleen is removed. The stomach should be carefully inspected to be sure every short gastric has been ligated.
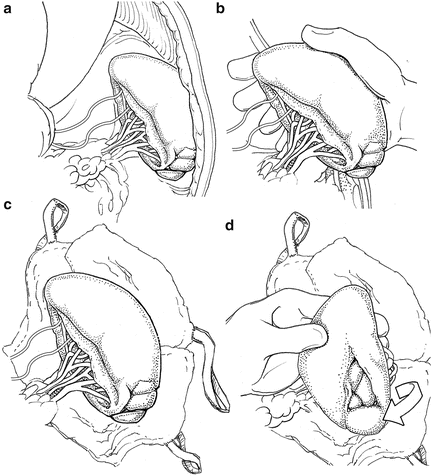

Fig. 12.1
(a) The spleen in situ. (b) Manual mobilization of the spleen from its attachments. (c) The spleen mobilized into the operative field resting on laparotomy pads. (d) Medial rotation of the spleen to control the hilum from its posterior aspect.
If the patient is in extremis, a one clamp splenectomy can be performed. An aortic vascular clamp is placed across the hilum and short gastric vessels. Care should be taken with this technique to avoid clamping the stomach. The clamp should be placed distal enough to avoid the tail of the pancreas as well (Fig. 12.2). Scissors are then used to remove the organ. The hilum and short gastric vessels can be controlled separately at this point by either ties or suture ligation. We use a combination of ties on all major vessels followed by additional suture ligature to reinforce the splenic artery and vein. Even if there is minimal concern for pancreatic injury from the rapid splenectomy, a closed suction drain can be left as energy transmitted to the injured spleen could also cause occult injury to the tail of the pancreas.
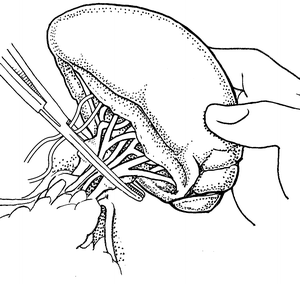

Fig. 12.2
Single clamp splenectomy with the clamp obtaining total vascular control of the hilum and short gastric vessels.
In a hemodynamically stable patient taken to the OR for laparotomy, if splenic injury is found, splenorrhaphy can be an option. In general, a stable patient with minimal blood loss and low-grade injury (grade 1 or 2) is a candidate for splenorrhaphy. However, if a patient has a severe traumatic brain injury such that recurrent hemorrhage may cause secondary brain injury or if the patient is coagulopathic or has comorbidities that may predispose to bleeding (such as chronic liver disease), splenectomy may be a better choice. We usually prefer splenectomy for higher-grade injuries as repeat laparotomy may be difficult if delayed bleeding occurs.
If splenorrhaphy is chosen, there are many options for surgical technique. Partial splenectomy can be done if the injury is limited to one pole. After mobilizing the spleen, temporary hemostasis may be obtained with finger pressure (Fig. 12.3). The corresponding vascular supply to that pole is ligated and divided. The splenic parenchyma is then cut with a scalpel (Fig. 12.4). The raw edge of the parenchyma is compressed with pledgeted horizontal mattress sutures. O-Vicryl or 2-0 Vicryl suture works well and some of the new bioabsorbable pledgets may reduce infectious risk (Fig. 12.5). Argon beam coagulation or placement of fibrin hemostatic sealant can be added to augment hemostasis. For smaller injuries, direct pledgeted suturing can be used to control bleeding from the spleen (Fig. 12.6). If the capsule of the spleen is torn and peeling off the spleen, but parenchymal damage is limited, either an omental patch or mesh wrap can be used (Fig. 12.7). For mesh, we prefer knitted Vicryl mesh soaked in a fibrin gel sealant. The mesh is wrapped around the area of injury (Fig. 12.8a). The sealant allows the mesh to adhere to the parenchyma and provides some additional hemostatic effect. Vicryl sutures can be used to tighten the mesh to compress the spleen and provide further hemostasis (Fig. 12.8b). The argon bean can be useful to weld the mesh to the spleen in some cases (Fig. 12.9). For very minor injuries, direct suture repair may work (Fig. 12.10).
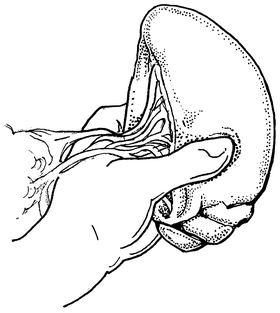
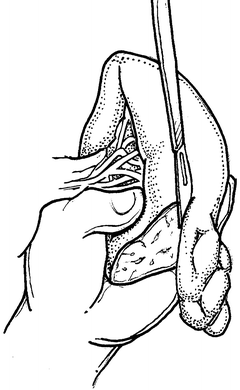
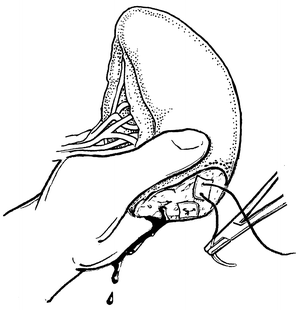
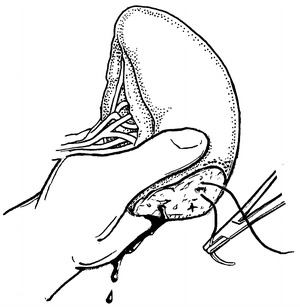
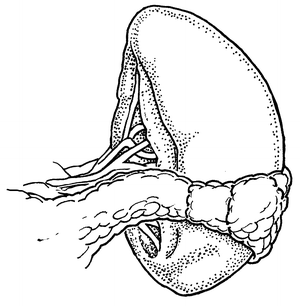

Fig. 12.3
Direct manual control of splenic bleeding in preparation for splenorrhaphy.

Fig. 12.4
Partial splenectomy for splenic salvage. Manual pressure is used to maintain control until the cut end is hemostatic.

Fig. 12.5
Mattress repair of the cut end of the spleen.

Fig. 12.6
Direct repair of vessels of cut end of spleen.

Fig. 12.7




Omentum wrapped around small splenic injury.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








