Differential medical diagnoses
Clinical clues
Potential tests
Gastrointestinal
Coeliac disease
Abdominal pain
Tissue transglutaminase blood testing
Inflammatory bowel disease
Abdominal pain, bloody diarrhoea. Family history
ESR, CRP and WCC. Discuss with gastroenterologist
Rheumatological
Systematic lupus erythematosus (SLE)
Joint involvement, rash
ESR, CRP and WCC. Consult paediatrician with expertise before autoimmune screen (risk of false positives)
Malignancies
Leukaemia
Hepatosplenomegaly, petechiae, fever, gum involvement
FBC
Discuss with paediatric haematologist for opinion and further tests (e.g. bone marrow aspirate)
Endocrinopathies
Thyroid disease (usually hyperthyroidism)
Tachycardia rather than bradycardia in AN and hypertension, family history
Thyroid function—may be abnormal in underweight per se
Diabetes mellitus
Ketones, excess thirst, family history
Random blood sugar. True concern: fasting blood sugar and HbA1c
Neurology
Space-occupying lesion
Headaches, behavioural changes, vomiting and poor growth. Abnormal fundoscopy or ataxia
MRI scan
Severe epilepsy
Paroxysmal changes
Consider EEG—preferably discuss with neurologist
It is beyond the scope of this chapter to discuss the potential chronic effects on growth and development in children and young people with eating disorders, which can be quite profound and lead to long-term stunting of growth (Nicholls et al. 2011a; Hudson and Court 2012) as well as poor bone mineral density leading to increased fracture risk (Jayasinghe et al. 2008; Faje et al. 2014). Children with significant underweight should ideally have their growth assessed and be seen by a paediatrician or endocrinologist with expertise in growth and puberty wherever possible. These chronic processes commence during the acute stages, and resolution or absence of acute findings does not necessarily mean that a child or adolescent is yet at a healthy weight (Hudson 2012). Readers are directed elsewhere for more detailed discussion of these topics (Nicholls et al. 2011a; Hudson and Court 2012; Rome et al. 2003). Paediatric expertise can also be valuable around the assessment of side effects and complications of psychotropic medications (Maayan and Correll 2011).
3.4 Assessing Medical Risk at Presentation in Children and Young People
Quantifying acute risk in children and young people with eating disorders is complex. Attempts to accurately guide risk management in children and young people is thwarted by a paucity of data, especially longitudinal data, to inform practice regarding risk parameters, both in terms of mortality and morbidity associated with risk at presentation.
An obvious outcome to be avoided is death. A meta-analysis of 42 studies reported the mortality rate from AN across all ages as 5.9 %, with half of deaths due to physical causes and half for mental health (e.g. suicide, substance misuse) (Sullivan 1995), again highlighting the importance of co-working between paediatric and mental health teams. Thankfully, deaths in children and adolescents with AN are rare—two national surveillance studies of early-onset eating disorders in the UK (Nicholls et al. 2011b) and Australia (Madden et al. 2009) found no deaths. Accurately measuring mortality is beset by reporting difficulties and the flux in incidence and prevalence over time, but one crude attempt using UK Office of National Statistics data from the 1990s estimates 5 deaths in 7 years in children and young people under 18 years of age (Wrate 2012). This should be put into the context of around 12 deaths per year from the more traditional “medical condition” diabetic ketoacidosis in the same age group (Hudson et al. 2012a).
At present, a number of consensus guidelines have been produced to help manage risk from starvation in AN principally focusing on recognising and managing medical instability particularly in the domains of cardiovascular, electrolyte and refeeding (Rome et al. 2003; Royal College of Psychiatrists 2011; Sylvester and Forman 2008; Beumont et al. 2004a). An additional function of these guidelines is to provide support and increase confidence of health professionals by providing a framework for practice. Here, we have focused on the Junior MARSIPAN guidelines, since these guidelines were derived from a synthesis of existing guidance. A summary of the framework for risk is presented in Table 3.2, a slightly modified version of the risk tables found in the Junior MARSIPAN guidelines with regard to QTc interval. Commentary on individual aspects of this risk framework is given below.
Table 3.2
Modified Junior MARSIPAN table
Red (high risk) | Amber (alert to high concern) | Green (moderate risk) | Blue (low risk) | |
|---|---|---|---|---|
BMI and weight | Percentage median BMI <70 % (approx. below 0.4th BMI centile) | Percentage median BMI 70–80 % (approx. between 2nd and 0.4th BMI centile) | Percentage median BMI 80–85 % (approx. 9th–2nd BMI centile) | Percentage median BMI > 85 % (approx. above 9th BMI centile) |
Recent loss of weight of 1 kg or more/week for 2 consecutive weeks | Recent loss of weight of 500–999 g/week for 2 consecutive weeks | Recent weight loss of up to 500 g/week for 2 consecutive weeks | No weight loss over the past 2 weeks | |
Cardiovascular health | Heart rate health (awake) < 40 bpm | Heart rate (awake) 40–50 bpm | Heart rate (awake) 50–60 bpm | Heart rate (awake) >60 bpm |
History of recurrent syncope; marked orthostatic changes (fall in systolic blood pressure of 20 mmHg or more, or below 0.4th–2nd centiles for age, or increase in heart rate of >30 bpm) | Sitting blood pressure: systolic <0.4th centile (84–98 mmHg depending on age and gender); diastolic <0.4th centile (35–40 mmHg depending on age and gender) | Sitting blood pressure: systolic <2nd centile (98–105 mmHg depending on age and gender); diastolic <2nd centile (40–45 mmHg depending on age and gender) | Normal sitting blood pressure for age and gender with reference to centile charts | |
Irregular heart rhythm (does not include sinus arrhythmia) | Occasional syncope; moderate orthostatic cardiovascular changes (fall in systolic blood pressure of 15 mmHg or more, or diastolic blood pressure fall of 10 mmHg or more within 3 min standing, or increase in heart rate of up to 30 bpm) | Pre-syncopal symptoms but normal orthostatic cardiovascular changes | Normal orthostatic cardiovascular changes | |
Cool peripheries; prolonged peripheral capillary refill time (normal central capillary refill time) | Normal heart rhythm | |||
ECG findings | Males and females <15 years | Males and females <15 years | Males and females <15 years | Males and females <15 years |
QTc > 460 ms | QTc > 460 ms | QTc 440–460 ms | QTc < 440 ms | |
Males (>15 years) | Males (>15 years) | Males (>15 years) | Males (>15 years) | |
QTc > 450 ms | QTc > 450 ms | QTc 430–450 ms | QTc < 430 ms | |
Females (>15 years) | Females (>15 years) | Females (>15 years) | Females (>15 years) | |
QTc > 460 ms | QTc > 460 ms | QTc 450–460 ms | QTc < 450 ms | |
With evidence of bradyarrhythmia or tachyarrhythmia (excludes sinus bradycardia and sinus arrhythmia); ECG evidence of biochemical abnormality | And taking medication known to prolong QTc interval, family history of prolonged QTc or sensorineural deafness | |||
Hydration status | Fluid refusal severe dehydration (10 %): reduced urine output, dry mouth, decreased skin turgor, sunken eyes, tachypnoea, tachycardiac | Severe fluid restriction moderate dehydration (5–10 %): reduced urine output, dry mouth, normal skin turgor, some tachypnoea, some tachycardia, peripheral oedema | Fluid restriction mild dehydration (<5 %): may have dry mouth or not clinically dehydrated but with concerns about risk of dehydration with negative fluid balance | Not clinically dehydrated |
Temperature | <35.5 °C tympanic or 35.0 °C axillary | <36 °C | ||
Biochemical abnormalities | Hypophosphataemia, hypokalaemia, hypoalbuminaemia, hypoglycaemia, hyponatraemia, hypocalcaemia | Hypophosphataemia, hypokalaemia, hyponatraemia, hypocalcaemia | ||
Disordered eating behaviours | Acute food refusal or estimated calorie intake 400–600 kcal per day | Severe restriction (less than 50 % of required intake), vomiting, purging with laxatives | Moderate restriction, bingeing | |
Engagement with management plan | Violent when parents try to limit behaviour or encourage food/fluid intake, parental violence in relation to feeding (hitting, force feeding) | Poor insight into eating problems, lacks motivation to tackle eating problems, resistance to changes required to gain weight, parents unable to implement meal plan advice given by healthcare providers | Some insight into eating problems, some motivation to tackle eating problems, ambivalent towards changes required to gain weight but not actively resisting | Some insight into eating problems, motivated to tackle eating problems, ambivalence towards changes required to gain weight not apparent in behaviour |
Activity and exercise | High levels of uncontrolled exercise in the context of malnutrition (>2 h/day) | Moderate levels of uncontrolled exercise in the context of malnutrition (>1 h/day) | Mild levels of uncontrolled exercise in the context of malnutrition (<1 h/day) | No uncontrolled exercise |
3.4.1 Risk from Degree of Underweight in Childhood and Adolescence
Weight, and the measurement of it, is a fundamental component of child and young people’s health, both in the assessment of acute illness and in surveillance of normal child health and chronic conditions. Paediatric teams spend much time measuring and referring to “underweight”, but the important concept of what underweight actually is is frequently forgotten! A useful definition of underweight is a low weight for height (conventionally using BMI), which is directly or indirectly detrimental to physical and psychological wellbeing, which in children potentially impacts on physical growth and development (including puberty), as well as neurocognitive development. In medical terms, detrimental features of underweight can be thought of as the acute features leading to medical instability which will be focused on here and the more chronic underweight or failure to thrive which may result in growth faltering.
For children, weight and height are traditionally compared to the population normal distribution using centiles, most commonly by plotting onto centile charts. This is in contrast to adults where standard body mass indices are used for overweight and underweight. In the UK, growth charts utilise the 1990 cross-sectional growth data taken from round 30,000 children of all ages and both sexes (Cole et al. 1995). Comparable data are available for other countries. More recently, body mass index (BMI: calculated as weight in Kg divided by the square of height in metres) has become the conventional way of measuring both underweight and overweight and both population-specific BMI centile charts and WHO global charts exist. The World Health Organization defines underweight using z-scores (standard deviations), below the mean for age and sex, with a BMI for age and sex −2 z-score (roughly equivalent to <2nd centile BMI) and severe underweight as under −3 z-score (Cole et al. 2007). In the eating disorder literature and clinical settings, an alternative measure called expected body weight or %median BMI is frequently used where a child’s BMI is expressed as a percentage of the median population BMI for age and sex (the 50th centile on a BMI growth chart). 90 %, 80 % and 70 % of the median BMI roughly equate to −1, −2 and −3 z-scores (or standard deviation scores (SDS)). % median BMI is frequently referred to as weight for height. This is potentially confusing, as there are a number of ways of calculating weight for height and should be avoided (Table 3.3).
Table 3.3
How to express underweight compared to population: centile and %median BMI (%mBMI) and WHO standards for underweight in children
Example: 9-year-old girl: weight 20.3 kg (0.9th centile), height 1.3 m (31st centile) BMI = Wt (kg)/Ht2 (m) = 12 (BMI centile <0.4th centile) BMI 50th centile for a child of the same age and sex is 16.1: thus, % of median BMI = 12/16.1 = 75 % of the median BMI (%mBMI) Using below classifications for this child : z-score = < −3 classified as severe thinness as per WHO and international grade (IOTF) -3 | |||
Measure | Threshold levels | Significance | Notes |
WHO underweight | −2 z-score | 2 standard dev below mean for age and sex | Approx. equivalent to 2nd centile BMI or 80 % median BMI |
WHO severe underweight | −3 z-scores | 3 standard dev below mean for age and sex | Less than 0.4th centile BMI and approx. equivalent to 70 % median BMI |
Although % median BMI and degree of underweight are an important measure of risk, it is likely a poor proxy for medical instability, at least for early-onset eating disorders. In a UK surveillance study, 40 % of children less than 13 years old presenting with an eating disorder with signs of medical instability had a BMI above the 2nd centile/-2SD/80 % median BMI (Hudson et al. 2012b). Thus, %BMI at presentation is poorly sensitive to medical instability. In contrast, %median BMI or degree of underweight is quite specific, with children at lower %median BMI usually having clinical evidence of medical instability. There are two possible explanations for the unreliability of degree of underweight at presentation as a marker of medical instability. Firstly, BMI is normally distributed, and by using % of the median or centiles for individuals, we are comparing individuals to the population average. Many children will normally be healthy above the average and many below. As an example, a child who was premorbid at the 80th centile for BMI and loses weight and presents at the 50th centile will be at 100 % median BMI but may have lost sufficient weight for him or her to be medically compromised. In contrast, a child normally on the 3rd centile for BMI (just above 80 % median BMI and by definition 3 % of children at any age will be at or below this point normally) might lose a smaller amount of weight to become below the 80 % median BMI position but not be medically affected. Added to this is the fact that the population norms are not racially sensitive, and therefore, application to non-Caucasian and mixed-race children is potentially misleading. The second important consideration is rate of weight loss. In the early-onset eating disorder study, rate of weight loss was associated with presence of medical instability at presentation independent of actual BMI or %BMI (Hudson et al. 2012b). Typically, overweight patients who develop eating disorders lose significant amounts of weight quickly to reach a healthy weight by population standards but have medical instability on examination.
The key messages are that those working with underweight should understand its measurement conceptually, and, irrespective of %median BMI or BMI centile at presentation, all children and young people should have a thorough medical examination at presentation to look for signs of medical compromise. Research on paediatricians in the UK suggests that this might not happen due to lack of knowledge (Hudson et al. 2013) and highlights the importance of co-working between medical and mental health professionals.
3.4.2 Risk Associated with Cardiovascular Parameters
The effects of starvation are seen most frequently in the cardiovascular system (Winick 1979; Peebles et al. 2006). As children starve, they rely on muscle metabolism to generate energy, and there is a paucity of protein for regeneration. The heart shrinks and this impacts the heart rate and its ability to function as a pump. Autopsies in starved children and young people show significantly reduced heart size (Kohn et al. 1998).
Bradycardia
Sinus bradycardia is a common finding in starvation and is thought to be a compensatory mechanism due to increased parasympathetic activity to reduce cardiac work and thus risk of heart failure (Casiero and Frishman 2006). Early experiments by the Jewish physicians in the Warsaw Ghetto during WWII on starved adults showed that administration of adrenaline had a blunted effect in raising the heart rate, illustrating the degree of parasympathetic block (Winick 1979). Bradycardia is usually asymptomatic and not a cause of concern, except of course that its presence is a clear demonstration of significant underweight. Various guidelines use degree of bradycardia as a guide for admission for medical stabilisation and nutritional rehabilitation (Beumont et al. 2004a; Rome et al. 2003). There is merit in this approach as intuitively a greater degree of bradycardia implies greater degree of starvation and a need for refeeding with close monitoring; however, there is no published evidence demonstrating greater risk with lower heart rate, and in addition, heart rate is normally distributed for normal children and shows significant variation by age and between individuals (Fleming et al. 2011). Clinicians should ensure that other important causes of bradycardia such as heart block are not present on an ECG. The bradycardia found in AN can be quite alarming for staff nursing patients if they are unfamiliar with it, especially as heart rates drop during sleep. When this occurs, heart rate will normally increase spontaneously during awakening or stimulation which can be a useful strategy for reassurance, although potentially unpleasant for patients.
Of greater significance is development of a tachycardia at low weight, as this may represent complications of prolonged QTc (see below) or development of the refeeding syndrome (see below).
Hypotension
Starvation in AN leads to decreased cardiac mass and reduced ventricular volumes (Casiero and Frishman 2006) which, combined with bradycardia, leads to reduced cardiac output. This can present clinically as hypotension, especially postural hypotension. As with degree of bradycardia, there is no evidence base for deciding what constitutes a significantly low blood pressure requiring an admission for medical stabilisation or a significant postural drop in blood pressure on standing. However, it is a more worrying physical sign in that, unlike bradycardia, it is a sign of decompensating cardiac function. Hypotension in children and young people should be defined with reference to published centiles (Jackson et al. 2007), using <0.2 or <2nd centile for either systolic or diastolic blood pressures as cut-offs for abnormality or, perhaps more importantly, where postural hypotension appears to be symptomatic. The caveat to this is that blood pressure, as for body mass index, is normally distributed within the population. By definition, some 2 % of the population will normally have a BP below the 2nd centile, and many children with an apparent normal blood pressure will be low relative to their typical blood pressure. As children and young people rarely have ever had a premorbid blood pressure recorded, it is usually not possible to have previous normal values to compare, though if they do exist, they should be sought so that a premorbid centile can be established.
Conventionally, a clinically significant postural drop in blood pressure is defined as 10–15 mmHg on standing or where it appears to be symptomatic, i.e. syncope on standing. Postural hypotension is concerning as it suggests poor systolic function. Postural hypotension may be associated with complications such as head injuries, and care must be taken in transportation to and from hospital beds, especially for toileting.
In an inpatient medical or emergency department setting, a very important consideration is iatrogenic risk associated with overzealous fluid management. A common mistake is for hypotension to be misinterpreted as shock, prompting generous fluid boluses that may initiate cardiac failure or pulmonary oedema. Dehydration and septic or haemorrhagic related shock can be differentiated from hypotension secondary to malnutrition by the presence of bradycardia, which is generally a terminal finding in the former in children and young people.
Prolonged QT Time (See Also Refeeding Below)
Published findings on prolonged QT time in AN are conflicting in the literature, with some studies finding it common, and others not at all (Casiero and Frishman 2006). It therefore remains controversial as to whether this is an important cause of death in AN. The safest approach is to perform a 12-lead ECG in all patients with weight loss and in those thought to be clinically underweight, especially in those with coexisting electrolyte abnormalities. The QT interval (see Fig. 3.1) is calculated as the distance from the start of the Q wave until the end of the T wave and represents the time taken for depolarisation and repolarisation of the ventricles. Prolonged QT time is associated with the polymorphic ventricular tachycardia known as torsades de pointes, a characteristic tachycardia where the QRS complexes appear to twist around the isoelectric line, which may lead to ventricular fibrillation (VF) or sudden cardiac death (Roden 1993). As the QT time shortens at slower heart rates and lengthens at longer heart rates, it is the most common convention to correct the QT time (corrected QT time or QTc) to a heart rate of 60 by dividing the QT by the square root of the RR interval (known as Bazett’s formula). Most ECG machines generate a rhythm strip from Lead II where the QTc can often be measured most easily and on which published normal ranges are based.
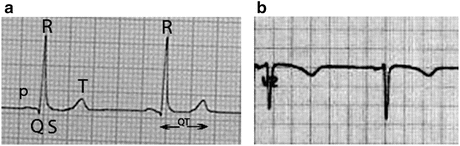

Fig. 3.1
The QT interval, corrected. (a) In this normal ECG, the pQRST complex is labelled. Each small square is 40 ms. The QT interval, from the Q wave to the end of the T wave, is 10 small squares, i.e. 400 ms. To calculate the QTc, divide the QT (in ms) by the square root of the R-R interval (in seconds). The R-R in this case is 24.5 × 40 ms = 980 ms, or 0.98 s. So the QTc is 400/√0.98 = 404.1 ms. (b) Readers are invited to calculate the same parameters on this second ECG. Note: each large square is 200 ms
Defining an abnormal QTc in children and young people is complex. QTc is normally distributed and differs between males and females in older adolescents and adults. Published data suggest that under 15 years of age (males and females), a borderline QTc is 440–460 ms and abnormal when >460 ms (Goldenberg et al. 2006). Over 15 years of age, a borderline QTc in males is 430–450 ms and abnormal >450 ms; in females, borderline is 450–460 ms, abnormal >460 ms (and in some populations, may be >470 ms) (Goldenberg et al. 2006; Bell and McLeod 2007). Because QTc is normally distributed, a number of normal children will be included by these criteria (Van Dorn et al. 2011; Bell and McLeod 2007). In general, when a prolonged or borderline QTc is found in patients, they should be discussed with a paediatrician with expertise in paediatric cardiology. It should be noted that the ranges mentioned here and included in Table 3.2 are a divergence from those cited in the Junior MARSIPAN guidelines, which the authors of this chapter think were too low and likely to lead to reduced specificity.
The QTc can be measured manually rather than relying upon automatically derived values by ECG machines, which can show considerable variation depending on the machine used (Kligfield et al. 2006). Common errors in calculating the QT interval are (1) using the incorrect units for each small square (one small square usually represents 0.04 s), (2) squaring rather than using the square root of the RR interval and (3) measuring from the end of the Q wave or beginning of the T wave. QTc time is frequently examined in paediatric postgraduate examinations, and paediatricians are usually proficient in its derivation. In contrast, studies have shown that psychiatrists may be less proficient (Solomons et al. 2008). This highlights the importance of pre-planning, training, and co-working and is another important role for the paediatric team in supporting mental health teams.
The appropriate management for prolonged QTc time is nutritional rehabilitation and correction of electrolyte abnormalities. Extra care must be taken when refeeding these patients (see below). It is important that all children and young people who are found to have prolonged QTc have their ECG checked once they are weight restored to ensure resolution. Persistent long QTc without underweight should prompt consideration of other causes of prolonged QTc, such as congenital (genetic) prolonged QTc, which is associated with sudden death and requires specialist cardiologist management. A congenital rather than acquired cause of prolonged QTc should be suspected when there are coexisting T-wave abnormalities.
Other Cardiac Complications
Mitral prolapse is not an uncommon finding on echocardiograms in severely underweight adolescents with AN (de Simone et al. 1994). Pericardial effusions have been reported in 20–35 % of adolescents with AN (Kastner et al. 2012; Docx et al. 2010). These tend to resolve with weight restoration, although they can complicate clinical progress. Although cardiac echocardiograms are not usually routinely indicated, a thorough clinic examination should be sought to find signs of the above. Abnormal echocardiographic findings should prompt repeat assessments upon weight restoration to ensure resolution and that they were not due to another aetiology. Such findings should also be discussed with a paediatrician with expertise in paediatric cardiology.
3.4.3 Dehydration
Presence of dehydration is variable in children and young people with AN, depending on whether restriction of oral intake has included fluids as well as food. Assessment and management of dehydration is a core skill of paediatricians. However malnutrition makes degree of hydration more challenging to assess as many of the tissue signs are unreliable (Mackenzie et al. 1989). History of fluid intake, losses and previous weights in children and young people who have been starved are also unreliable. Therefore, clinicians may need to rely more on urea and electrolytes. Fluid rehydration is always safer orally rather than via an intravenous route, so if dehydration is prominent, it is better to rehydrate with oral rehydration solution in an inpatient setting for 24 h, if this is tolerated and there is no shock present. As this contains few calories, it is usually considered acceptable by patients with AN. Presence of hypernatraemia should prompt slower rates of rehydration, and if corrected intravenously, isotonic fluids (e.g. 0.9 % NaCl) should be used. Dieticians should also factor fluid requirements into the meal plan as part of a team approach.
3.4.4 Hypothermia
Despite being relatively common, surprisingly, little is known about both the aetiology and consequences of hypothermia in AN. It is likely a combination of low body fat content (leading to decreased capacity for insulation), low metabolic rate in starvation, central hypothalamic dysfunction and also potentially thyroid abnormalities. From a risk perspective, degree of hypothermia reflects severity of underweight, although again there is little evidence for what constitutes risk. Whether or not patients with hypothermia are at increased risk of pathologies relating to hypothermic illness is also unclear. The best practice is to provide nutrition to recover.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






