Fig. 21.1
Full-thickness ischemia of stoma
While edema and venous engorgement in the immediate postoperative period is common, this was a case of stoma necrosis, which is one of the more common early complications following creation of stomas which also include stoma retraction, dermatitis, leakage, and high output dehydration. Stoma necrosis is characterized by discoloration of the mucosa ranging from dusky blue/purple to gray and black as ischemia progresses and poor or absent blood flow from raw surfaces. Stoma necrosis occurs in 1–20 % of colostomies and 1–10 % of ileostomies [4] and is more frequently observed in obese patients and following emergency surgeries [3]. Necrosis is due to inadequate perfusion and usually presents within the first few postoperative days [4, 5]. Treatment includes fluid resuscitation, reversal of hypotension, and hypoxia to improve overall tissue perfusion. Adequate bowel decompression with a nasogastric tube may reduce abdominal girth and tension on the bowel mesentery. Partial ischemia involving mucosa only and not extending below the level of the fascia (Fig. 21.2) may be managed expectantly, although risk of long-term stoma stricture and retraction is increased [1, 5]. Full-thickness necrosis and necrosis extending below the level of the fascia require immediate surgical revision to prevent perforation and peritoneal contamination [3, 5]. To determine whether tissue loss is full thickness, a dry gauze pad can be used to gently debride devitalized superficial tissue, underlying submucosa that is pink and bleeding is reassuring. To evaluate depth of ischemia, a small lubricated test tube can be inserted into the lumen of the stoma and a flashlight used to illuminate the interior to assess tissue viability below the skin and above the fascia.
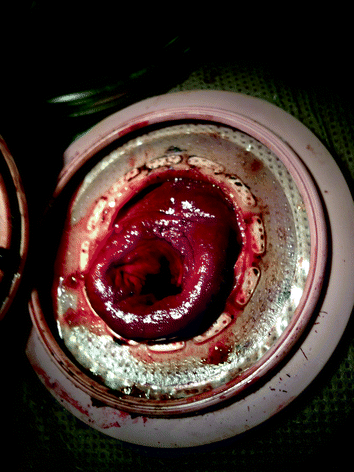

Fig. 21.2
Partial ischemia of superficial (above skin) stoma
Because the necrosis in this case was full thickness and complete, I advised a return to the operating room for revision. In order to maximize success of revision, the patient’s physiology should be optimized with resuscitation, nasogastric decompression, and reversal of any coagulopathy, hypothermia and acidosis. Additionally, appropriate antimicrobial coverage should be anticipated and dosed to ensure therapeutic circulating levels at the time of the procedure. If not utilized previously, an enterostomal therapist should be consulted to mark the skin for optimal stoma placement in case stoma relocation is required. If no enterostomal therapist is available, or in the case of emergency surgery, the optimal site for stoma placement is generally within the rectus sheath at the apex of the subumbilical fat roll, approximately two-thirds of the way along an imaginary line from anterior superior iliac spine and the umbilicus [3, 6].
The most common causes of stoma necrosis are tension upon the stoma due to inadequate mobilization or short bowel mesentery, external compression of mesentery by an overly small abdominal wall opening, and over trimming or ligation of the mesentery. In the operating room following a general abdominal inspection for other pathology and evacuation of any bloody, purulent or feculent material attention should be turned to the bowel. For descending colostomies as was the case for this patient, complete mobilization along the left lateral white line of Toldt and release of the splenic flexure are imperative. Particularly in obese patients who tend to have a short and fatty mesentery, the mobilization of the mesentery off of the retroperitoneum should extend to the midline medial to the ligament of Treitz. Additional length can be obtained by dissecting the distal transverse colon from its attachments to the greater curvature of the stomach and removing a portion of the greater omentum. The goal of mobilization should be easy movement of the bowel through the abdominal wall and protrusion of at least 3–4 cm of bowel above the skin without any tension. In extreme circumstances, this may require ligation of the inferior mesenteric vein at the lower border of the pancreas, and if further length is still required this can be followed with ligation of the inferior mesenteric artery (IMA) near its origin releasing the medial and most proximal tether on the colonic mesentery [1, 6]. It is essential to preserve the marginal artery and the collateral blood supply to the descending colon when required to ligate the IMA. When artificially foreshortened due to peritoneal inflammation, additional length can be obtained by pie-crusting of the mesentery creating several staggered partial thickness incisions in the thickened overlying peritoneum without disrupting the underlying vasculature.
After establishing adequate length for stoma creation, attention is then turned to the abdominal wall opening. In the case of my colleague’s patient, the stoma aperture had already been created but when creating a de novo aperture it is important to localize the opening within the rectus sheath and limit the aperture size. Location within the rectus muscle, as opposed to lateral to the rectus sheath, has been associated with reduced risk of parastomal hernia and stoma prolapse [6]. Several studies have also found that an aperture size greater than 2.5–3.5 cm is a risk factor for parastomal hernia and that every millimeter increase in aperture diameter increases risk of hernia by 10 % [1, 7]. However, in obese patients and those with a very thick mesentery, the anterior and posterior rectus sheaths should be divided widely in a vertical fashion and the rectus muscle split widely in a muscle-sparing fashion; only the skin aperture should be left small enough to accommodate the bowel alone. This allows for easy passage of the stoma without damage to the vascular supply, and the large incision can accommodate the fatty mesentery of the left colon without causing external compression of the mesentery and vascular compromise. Once the bowel is mobilized such that 3–4 cm of bowel is easily and freely externalized without tension, the anterior and posterior sheaths can be partially closed around the bowel on the antimesenteric side of the aperture. Care should be taken during externalization of the bowel and closure of the abdominal wall defect around the stoma that no twisting or kinking of the bowel or volvulization can occur.
Parastomal herniation is a particular concern for this patient given the patient’s risk factors of obesity, the original emergent nature of the procedure, and that it is colostomy, as these have higher rates of hernia formation compared to ileostomies [1, 3, 7]. Other risk factors for hernia formation include advanced age, poor nutrition, malignancy, steroid use, and end stomas. In addition to localizing the stoma exit to the rectus sheath and limiting the size of the aperture if possible, consideration should be given to placement of preperitoneal or sublay mesh in order to reduce the risk of hernia formation. There is good evidence including 3 randomized controlled trials demonstrating a significant risk reduction in hernia formation (RR 0.23, p = 0.02) and need for surgical hernia repair (RR 0.13, p = 0.05) when using either biological or prosthetic mesh reinforcement of the stoma aperture [8]. These studies also demonstrated no increase in rates of infections or other stoma-related morbidity and mortality. Because of the patient’s risk factors, the need for early revision, and because it is the experience at our medical center that follow up for stoma closure in our patient population is unreliable, in this case I advised reinforcement with biological mesh. Although rates of infections have not been shown to be increased with prosthetic mesh when compared to bioprosthetics [1, 7], we feel that risk of infection in frankly contaminated fields and in patients who are immunocompromised because of critical illness is too great to use prosthetic material and favor biological mesh such as FlexHD®, AlloDerm®, StratticeTM, or Permacol®. When utilized, it should ideally be placed in the preperitoneal space, although if coagulopathy makes extensive dissection undesirable, an underlay position can be used. The mesh should be large enough to create a 4- to 5-cm overlap and a keyhole configuration or crosscut aperture can be used to accommodate the stoma. The mesh should be secured circumferentially around the lateral borders. I recommend #1 or 0 Ethibond and space the sutures approximately 2–3 cm apart.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








