KEY POINTS
1. Core temperature (nasopharyngeal or tympanic membrane) should be greater than 36°C before terminating cardiopulmonary bypass (CPB). However, the nasopharyngeal temperature should not exceed 37°C, as this will increase the risk of postoperative central nervous system dysfunction. Using nasopharyngeal temperature to avoid hyperthermia and the rectal/bladder temperature to avoid underwarming may be the safest technique.
2. Visualization of the heart, directly as well as with transesophageal echocardiography (TEE), is important before terminating CPB.
3. “The first attempt to terminate CPB is the best one.” Optimize all parameters before CPB termination.
4. When terminating CPB, left ventricular failure is suggested by a decreased pulse pressure.
5. Vasoplegic syndrome is a severe form of post-CPB vasodilation characterized by low arterial pressure, normal to high cardiac output (CO), normal right-side filling pressures, low systemic vascular resistance (SVR) which is refractory to pressor therapy.
6. When evaluating hypoxemia after CPB, the possibility of a right-to-left shunt through a patent foramen ovale must be considered and evaluated with TEE.
7. New onset renal dysfunction requiring dialysis after CPB will increase mortality almost eightfold.

TERMINATING CPB REQUIRES THE ANESTHESIOLOGIST to apply the basic tenets of cardiovascular physiology and pharmacology. The goal is a smooth transition from the mechanical pump back to the heart as the source of blood flow. Weaning from the pump involves optimizing cardiovascular variables including preload, afterload, heart rate (HR), conduction, contractility, and the O2 supply–demand ratio, as in the pre-CPB period. However, the time period for optimization is compressed to minutes or seconds, and decisions must be made quickly to avoid myocardial injury or damage to the other major organ systems.
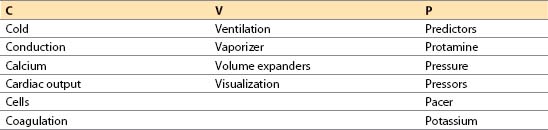
I. Preparation for termination of bypass: CVP mnemonic.
The major objectives in preparing for termination of CPB can be remembered with the aid of the mnemonic CVP:
1
A. Cold. Core temperature (nasopharyngeal or tympanic membrane) should be greater than 36°C before terminating CPB. Rectal or bladder temperature should be at least 35 to 36°C [1]. Ending CPB when cold causes prolonged hypothermia from equilibration of the cooler, vessel-poor group with the warmer and better perfused vessel-rich group. Nasopharyngeal temperature correlates with brain temperature but may be artificially elevated during rapid rewarming and should not be used for determining the temperature at which CPB is discontinued unless it has been stable for 20 to 30 min. Venous return temperature can be used in a similar manner to help confirm core temperature. The nasopharyngeal temperature should not exceed 37°C, as this will increase the risk of postoperative central nervous system dysfunction. Using nasopharyngeal temperature to avoid hyperthermia and the rectal/bladder temperature to avoid under-warming may be the safest technique.
B. Conduction. Cardiac rate and rhythm must be controlled as follows:
1. Rate
a. HR of 80 to 100 beats/min often is needed for adequate CO post-CPB because of reduced ventricular compliance and inability to increase stroke volume. In coronary artery bypass graft (CABG) procedures, complete revascularization allows a higher rate (80 to 100 beats/min) after CPB, with less risk of ischemia than before CPB. Patients with severely limited stroke volume (aneurysmectomy or after ventricular remodeling) may require even higher rates.
b. Sinus bradycardia may be treated with atropine or an inotropic drug, but epicardial pacing is more reliable.
c. Sinus tachycardia of more than 120 beats/min should be treated before termination of CPB. Often the act of “filling the heart” and increasing preload will reflexively decrease the HR to an acceptable level. Other etiologies of increased HR must be addressed. Common etiologies include:
(1) Hypoxia
(2) Hypercapnia
(3) Medications (inotropes, pancuronium, scopolamine)
(4) Light anesthesia, awareness
(a) “Fast track” anesthesia with its lower medication dosing schedule requires special attention to this complication. An additional dose of narcotic and benzodiazepine, or hypnotic (propofol infusion) should be considered during the rewarming period if tachycardia is present. BIS or depth of anesthesia monitors may be helpful in guiding therapy.
(5) Anemia
(6) Ischemia: ST and T-wave changes indicative of ischemia should be treated and the surgeon should be notified. A nitroglycerin (NTG) infusion and/or an increase in the perfusion pressure often improves the situation. Refractory causes include residual air or graft occlusions. If coronary air is suspected, briefly increasing the perfusion pressure to a mean of 90 mm Hg may improve the situation.
2. Rhythm
a. Normal sinus rhythm is preferable. In patients with poorly compliant, thick-walled ventricles (associated with aortic stenosis, hypertension, or ischemia), the atrial “kick” may contribute up to 40% of CO, so attaining synchronized atrial contraction (sinus rhythm, atrial or atrioventricular [AV] sequential pacing) is very important before attempting CPB termination. This may require a discussion with the surgeon if they feel ventricular pacing alone is adequate. Atrial pacing is acceptable if there is no AV block, but often atrial and ventricular leads are needed.
b. Supraventricular tachycardias (HR greater than 120 beats/min) such as regular narrow-QRS atrial flutter and atrial fibrillation, should be cardioverted with synchronized internal cardioversion before terminating CPB.
c. Esmolol, verapamil, amiodarone, or adenosine may be used to chemically cardiovert or to control the ventricular response rate. A decrease in contractility is seen with some agents.
d. Third-degree AV block requires pacing, although atropine occasionally may be effective.
e. Ventricular dysrhythmias are treated as indicated (see Chapter 2).
C. Calcium. Calcium should be immediately available to treat hypocalcemia and hyperkalemia, which commonly occur after CPB. However, the routine administration of calcium post-CPB is not recommended.
1. Mechanism of action. Most studies suggest that calcium produces an elevation in SVR when the ionized Ca2+ level is in the low–normal range or higher [2]. Despite this increase in afterload, contractility is maintained. At very low ionized calcium levels (<0.8 mM), contractility is increased by calcium administration. Elevating calcium levels will also help counteract the dysrhythmogenic and negative inotropic actions of hyperkalemia. The usual dose is 5 to 15 mg/kg of CaCl.
2. Measurement. Ionized Ca2+ levels should be evaluated after rewarming to help direct therapy. Citrated blood cardioplegia reperfusion solutions can lower blood Ca2+ levels substantially. The usual range is 1 to 1.3 mmol/dL. Calcium levels are affected by pH: Low pH will increase Ca2+ levels, whereas elevated pH will decrease Ca2+ levels. Correction of pH should be attempted before treating abnormal values.
3. Risks of calcium administration
a. Patients taking digoxin may experience life-threatening dysrhythmias.
b. Inhibition of the hemodynamic action of inotropes (e.g., epinephrine, dobutamine) has been reported.
c. Coronary spasm might occur in rare susceptible patients.
d. Augmentation of reperfusion injury is possible. Calcium administration should wait until 15 min after aortic cross-clamp release.
D. Cardiac output. Evaluating cardiac function is vital after CPB. CO may be obtained from a pulmonary artery (PA) catheter or contractility may be estimated by using TEE. If a continuous CO PA catheter is used, it may take more than 3 min to obtain the first CO after CPB. If the patient is stable, this is acceptable; if not, the equipment for a manual determination should be used initially.
E. Cells
1. The hemoglobin concentration should be measured after rewarming. If it is less than 6.5 to 7 g/dL before terminating CPB, blood administration should be considered to maintain O2-carrying capacity after CPB. If the venous reservoir contains a large amount of blood, this blood may be concentrated by a cell saver and given back to the patient after CPB, which could preclude the need for a blood transfusion. Patients with residual coronary stenoses, anticipated low CO, or end-organ damage may benefit from even higher hemo-globin concentrations.
2. Two units of packed red blood cells (PRBCs) should be immediately available for use once the CPB pump volume is exhausted. If excessive bleeding is anticipated (see Section F below), then additional units should be on hand.
F. Coagulation. Anticipation of possible coagulation abnormalities is necessary prior to discontinuation of CPB. Blood components should be administered only after CPB when the heparin has been reversed and all surgical repairs are complete. Blood component therapy should be guided by the clinical situation and laboratory findings (e.g., thromboelastogram, prothrombin time, partial thromboplastin time, platelet count).
1. Patients at risk include:
a. Patients taking platelet inhibitors (clopidogrel, prasugrel, ticlopidine, aspirin) [3]
b. Patients having emergency surgery and who have been exposed to:
(1) Thrombolytic agents (alteplase, tenecteplase)
(2) Antiplatelet glycoprotein IIb/IIIa agents (abciximab, eptifibatide, tirofiban)
(3) Direct thrombin inhibitors (bivalirudin, dabigatran, argatroban)
(4) Coumadin
c. Patients with chronic renal failure
d. Long “pump run,” e.g., redo or complex operation
e. Low body mass index (BMI)
f. Extreme hypothermia on CPB
g. Excessive bleeding with previous CABG [4]
2. Platelets should be available if indicated (as above).
3. Desmopressin acetate (DDAVP) can be used to increase platelet aggregation in patients with chronic renal failure, acquired von Willebrand disease which occurs with aortic stenosis, or other platelet abnormalities. In patients without pre-existing platelet abnormalities, DDAVP has little effect on blood loss or replacement in CABG patients but may be effective in open-chamber surgery (see Section VI.B.3.b).
4. Fresh frozen plasma or cryoprecipitate should be available if indicated for the treatment of appropriate factor deficiencies.
5. Factor concentrates (specifically rVIIA and prothrombin complex concentrates [PCCs]) have been used in cases of severe refractory bleeding. Possible complications are related to increased risk of thrombosis (coronary occlusion, graft occlusion, stroke).
G. Ventilation
1. Adequate oxygenation and ventilation while the patient is on CPB must be ensured by checking arterial and venous blood gas measurements at routine intervals. Arterial pH should be between 7.3 and 7.5 at normothermia before CPB separation.
2. The lungs should be re-expanded with two to three sustained breaths (15 to 20 s each) to a peak pressure of 30 cm H2O with visual confirmation of bilateral lung expansion and resolution of atelectasis. In patients with internal mammary artery grafts, care must be taken to prevent lung overdistention, which may cause graft avulsion. Coordinate this maneuver with the surgical team. An estimate of lung compliance should be made (see Section 7). The surgeon may need to evacuate any hemothorax or pneumothorax.
3. Inspired oxygen fraction (FiO2) should be 100%. If air was used during CPB to prevent atelectasis, it should be discontinued. Nitrous oxide should never be used during or after cannulation to avoid increasing the size of air emboli.
4. Confirm pulse oximeter is working once pulsatile flow returns. The pulse oximeter may not work, however, despite pulsatile flow in a patient who is still cold and peripherally vasoconstricted.
5. All airway monitors should be on line (apnea, PIP, FiO2, end-tidal CO2).
6. Mechanical ventilation must be started before an attempt to terminate CPB. The timing for commencement of mechanical ventilation while the patient is still on CPB is controversial. Some practitioners believe that ventilation should begin when arterial or pulmonary pulsatile blood flow resumes in order to avoid hypoxemia. However, this may not be necessary in normothermic, nearly full-flow bypass and may cause severe respiratory alkalosis of pulmonary venous blood. The pulse oximeter or the CPB circuit venous oxygen tension also can be used to assess the need for ventilation during partial CPB.
7. Auscultation of breath sounds will confirm air movement and may reveal wheezing, rales, or rhonchi. Visual confirmation of bilateral lung expansion is important. Appropriate treatment (suctioning, bronchodilators) should be instituted before terminating CPB. Bronchoscopy may occasionally be needed.
H. Vaporizer. Inhalation agents used during CPB for blood pressure (BP) control ordinarily should be turned down or off at least 10 min before terminating CPB. These agents will decrease contractility and confuse the etiology of myocardial dysfunction postbypass.
I. Volume expanders. Colloid or crystalloid solution should be available to increase preload if blood products are not indicated. Hetastarch may be contraindicated if excessive bleeding is anticipated or with impaired renal function.
2
J. Visualization of the heart is important before terminating CPB. Primarily. Primarily the right atrium and ventricle are visible in the chest. TEE is helpful in permitting a detailed examination. It is possible to evaluate the following parameters:
1. Contractility. An experienced observer can often estimate contractility by just looking at the heart in the chest. Wall-motion abnormalities from ischemia or infarct should be compared to pre-CPB observations.
2. Distention of the chambers can be seen with both methods.
3. Residual air in left-sided structures (e.g., left atrium [LA], left ventricle [LV], pulmonary veins). Inspection during and after ventilation will confirm the location of residual air.
4. Conduction. Direct observation of the atria and ventricles can often help differentiate dysrhythmias easier than using the electrocardiogram (ECG). Visualization of the RA appendage may prove especially helpful in this regard. A four-chamber view is most helpful.
5. Valvular function or perivalvular leaks should be identified before attempting CPB termination so that repair can be accomplished if needed.
K. Predictors and factors contributing to adverse cardiovascular outcome
1. Assess the patient’s risk for difficult weaning from CPB. Risk factors that can be identified before terminating CPB include [5]:
a. Preoperative ejection fraction (EF) less than 45% or diastolic dysfunction
b. Renal disease—increased morbidity and mortality with increasing creatinine
c. Female patient undergoing CABG (tendency for incomplete revascularization due to smaller more diseased coronary arteries)
d. Elderly patient
e. Congestive heart failure (usually related to valvular or myocardial dysfunction)
f. Emergent surgery
(1) Ongoing ischemia or evolving infarct
(2) Failed closed intervention (angioplasty/stent/valvuloplasty)
g. Prolonged CPB duration (more than 2 to 3 hrs)
h. Inadequate surgical repair
(1) Incomplete coronary revascularization
(a) Small vessels (not graftable or poor “runoff”)
(b) Distal disease (especially in diabetic patients)
(2) Valvular disease
(a) Valve replacement with very small valve (high transvalvular pressure gradient post-CPB)
(b) Suboptimal valve repair (residual regurgitation or stenosis)
i. Incomplete myocardial preservation during cross-clamping
(1) ECG not asystolic (incomplete diastolic arrest)
(2) Prolonged ventricular fibrillation before cross-clamping
(3) Warm myocardium
(a) LV hypertrophy (incomplete cardioplegia)
(b) High-grade coronary stenoses (no cardioplegia to that area of heart)
(c) Choice of grafting order (grafts should be performed first in an area of the heart served by a high-grade lesion in the absence of retrograde cardioplegia, so cardioplegia may be infused early)
(d) Noncoronary collateral flow washing out cardioplegia
(e) Poor LV venting causing cardiac distention (aortic insufficiency if using anterograde cardioplegia)
(f) Inadequate topical cooling
j. Prolonged ventricular failure
k. Impaired myocardial perfusion before and after cross-clamping
(1) Low perfusion pressure on CPB (less than 50 mm Hg)
(2) Ventricular distention
(3) Emboli (air, clot, particulate)
(a) From ventriculotomy or improper de-airing of coronary grafts
2. Additional preparations for high-risk patients
a. One common practice is to have a syringe of ephedrine (5 mg/mL) or dilute epinephrine prepared (4 to 10 μg/mL). Boluses can be used until a decision is made regarding the need for further inotropes.
b. Discuss the need for additional invasive monitoring with the surgeon (i.e., LA or central aortic catheter).
c. Check for immediate availability of other inotropic or vasoactive medications: epinephrine, dopamine, milrinone, norepinephrine, nitric oxide, or inhaled epoprostenol (Flolan).
d. As appropriate to the anticipated level of difficulty separating from CPB, check for immediate availability of an intra-aortic balloon pump (IABP). Consider placement of a femoral arterial catheter prebypass to facilitate its rapid insertion and possibly for improved BP monitoring.
e. Consider starting an inotropic infusion or the IABP before terminating CPB in patients with poor contractility. Note that the Frank–Starling law implies that an empty heart will not beat very forcefully. Often a sluggishly contracting heart will start to “snap” once it is filled.
3
f. “The first attempt at terminating CPB is the best one.” Optimizing all parameters before CPB termination is strongly advised. If in doubt, start an inotrope. Pre-emptive use of milrinone has been shown to improve cardiac function during and after cardiac surgery. A milrinone bolus without an infusion can be sufficient in marginal candidates [6].
g. Ischemic preconditioning/postconditioning. The heart will react to a low level ischemic stress and subsequent exposure to free radicals by becoming more “resistant” to further ischemic injury. This can be attempted in the OR [7,8].
(1) Inhalational agents (isoflurane/sevoflurane have been most studied) can mimic this effect.
(a) This can be accomplished by using the agent at 1 to 2.5 MAC for 5 to 10 min after initiating CPB but before the aortic cross-clamp has been placed. Then a 10-min washout occurs prior to starting cardioplegia.
(b) Others suggest using sevoflurane pre-CPB, during CPB, and post-CPB instead of using a propofol infusion [9].
(2) Ketamine, nicorandil, and the “statins” have also been studied with beneficial results [10].
(3) Postischemic conditioning by brief sequential ischemia and reperfusion episodes has also been suggested in this setting.
L. Protamine. The protamine dose should be calculated and drawn up in a syringe or should be ready as an infusion. Premature use of protamine is catastrophic. Protamine should be prominently labeled and should not be placed where routine medications are stored to avoid accidental use. The surgeon, anesthesiologist, and perfusionist must all coordinate the use of this medication.
M. Pressure. Check the calibration and zero level of all transducers before terminating CPB.
1. Arterial pressure. Recognize that radial artery catheters may underestimate central aortic pressure following rewarming [11]. Femoral artery catheters do not share this limitation. An aortic root vent, if present, may be connected to a transducer also. If the radial arterial catheter is not functioning, a needle placed in the aorta or aortic cannula can be transduced during and after termination of CPB until the cannula is removed.
2. PA pressure. Ensure that the catheter has not migrated distally to a wedge position. Often the PA catheter must be withdrawn 3 to 5 cm even if this was done at CPB initiation.
N. Pressors and inotropes
1. Medications that are likely to be used should be readily available, including a vasodilator (e.g., NTG, nitroprusside) and a potent inotropic agent (e.g., dopamine, dobutamine, epinephrine, milrinone).
2. NTG and phenylephrine should always be available to infuse after CPB as they are almost always used. Some practitioners use prophylactic NTG infusion (approximately 25 to 50 μg/min) for all coronary revascularization procedures to prevent coronary spasm and to enhance non-coronary collateral flow in cases of incomplete revascularization. It also can be used as a venodilator to allow additional CPB pump volume to be infused in patients after CPB.
3. Volumetric infusion pumps deliver vasoactive substances with the highest accuracy and reproducibility.
O. Pacer. An external pacemaker should be in the room, checked, and set to the initial settings by the anesthesiologist. A pacemaker often is needed for treatment of relative bradycardia or asystole. In patients with heart block, an AV sequential pacemaker is strongly advised to retain a synchronized atrial contraction. Use of a DDD pacer, when available, is recommended. Temporary biventricular pacing in patients with reduced EF is used in some centers.
P. Potassium. Blood chemistries should be checked before terminating CPB.
1. Hyperkalemia may induce conduction abnormalities and decreases in contractility. It is more common after long pump runs when large amounts of cardioplegia solution are used and absorbed, especially in patients with renal dysfunction.
2. Hypokalemia can cause dysrhythmias and should be treated if less than 3.5 mEq/L and there is adequate urine output after CPB.
3. Glucose levels should be checked and treatment undertaken for hyperglycemia in all patients, not just diabetic patients. Hyperglycemia may contribute to central nervous system dysfunction, poor wound healing, and cardiac morbidity. The optimal glucose level is controversial. Some advocate “aggressive treatment” and suggest a glucose level of 110 mg/dL. Most authors try to maintain a level less than 180 mg/mL due to concerns that more aggressive control may lead to complications related to hypoglycemia and possibly higher mortality [12].
4. Ionized Ca2+ levels are discussed in Section I.C above.
5. Other electrolytes should be evaluated as needed. In particular, low levels of magnesium are common after CPB and have been associated with dysrhythmias, coronary vasospasm, and postoperative hypertension. Magnesium (2 to 4 g) can be administered into the pump prior to emergence from CPB [13].
II. Sequence of events immediately before terminating CPB.
Weaning from bypass describes the transition from total CPB to a final condition in which the heart provides 100% of the work. The transition should be gradual, recognizing that cardiac function post-CPB is not usually normal. At times, though, cardiac function may be improved after bypass if ischemia is relieved or valvular dysfunction repaired.
A. Final checklist before terminating CPB
1. Confirm
a. Ventilation
(1) Lungs are ventilated with 100% O2, visual confirmation, and ETCO2 present.
(2) Ventilatory alarms are enabled.
(3) Breath sounds and heart tones are heard via the esophageal stethoscope.
b. The patient is sufficiently rewarmed.
c. The heart, great vessels, and grafts have been properly de-aired.
d. The patient is in optimal metabolic condition.
e. All equipment and medications are ready.
2. Do not proceed until these criteria have been met.
3. Weaning from CPB requires the utmost concentration and vigilance by the anesthesiologist, and all distractions should be eliminated. Turn the music down and limit extraneous conversations.
B. What to look at during weaning. Key information can be obtained from four sources: the invasive pressure display, the heart itself, the TEE, and the ECG.
1. Invasive pressure display
a. Pressure waveforms (arterial, central venous pressure [CVP], and PA or LA, if used) are best displayed using overlapping traces, and there are some benefits to the use of an identical scale as well. Advantages of this display format include the following:
(1) Coronary perfusion pressure is graphically depicted as the vertical height between the arterial diastolic pressure and the filling pressure (PA diastolic or LA mean) during diastole.
(2) The vertical separation between the PA mean and CVP waveforms estimates right ventricular (RV) work.
(3) The slope of the rise in central aortic pressure during systole may give some indication of LV contractility and is most easily appreciated if the waveform is not compressed.
(4) Valvular regurgitation can be diagnosed by examining CVP, pulmonary capillary wedge pressure (PCWP), or LA waveforms (e.g., mitral regurgitation may produce V waves in LA and PCWP tracings) as well as by TEE.
b. Arterial pressure. The systolic and mean systemic arterial pressures should be checked continuously.
(1) The systolic pressure describes the pressure generated by the heart’s own contraction.
(2) Before CPB separation, the mean pressure describes the work performed by the bypass pump and the vascular tone. After separation, it reflects the cardiac work and vascular tone.
(3) The diastolic pressure reflects vascular tone and gives an indication of coronary perfusion pressure.
(4) The pulse pressure reflects the mechanical work done by the heart. As the heart assumes more of the circulatory work, this pressure difference increases. LV failure is suggested by a decreased pulse pressure.
4
(5) Difficulty in weaning (poor LV function) may be reflected by a low pulse pressure or systolic minus mean pressure difference in the presence of high atrial filling pressures when the venous return line is partially occluded.
(6) It is important to remember that a radial artery catheter may not be accurate following CPB. During the first 30 min after CPB, the radial artery tends to underestimate both the systolic and mean central aortic pressures. The surgeon can often confirm that there is a pressure difference by palpating the aorta. Clinically significant radial artery hypotension should be confirmed by a noninvasive BP reading or with a central aortic or femoral artery pressure measurement before treatment or resumption of CPB.
c. CVP. This provides an index of right heart filling before and during weaning.
(1) Inspection of the heart visually or by TEE provides valuable information about contractility, wall-motion abnormalities, conduction, preload, valvular function, and quality of surgical repair.
(2) ECG changes, such as heart block, dysrhythmias, or ischemia, occur frequently, mandating frequent examination.
(3) TEE. The mid-papillary transverse view is best for obtaining EF, filling parameters, and regional wall motion abnormalities. The four-chamber view can reveal valvular function and conduction abnormalities.
(4) Ventilation and oxygenation. Routine airway management issues as well as problems in the other major organ systems must not be overlooked. The partial pressure of carbon dioxide (PaCO2) should be kept at or below 40 mm Hg in the post-CPB period. Minor elevations in PaCO2 can increase pulmonary vascular resistance (PVR) significantly. This is most important when RV failure is noted.
III. Sequence of events during weaning from CPB
A. Step 1: Impeding venous return to the pump
1. Consequences of partial venous occlusion. Slowly the venous line is partially occluded (by the surgeon or perfusionist). This increase in venous line resistance causes right atrial pressure to rise and diverts blood flow through the tricuspid valve into the RV instead of drainage into the pump. According to the Frank–Starling law, CO increases as preload rises; therefore, the heart begins to eject blood more forcefully as the heart fills and enlarges.
2. Preload. The amount of venous line occlusion is adjusted carefully to attain and maintain a certain optimal preload or LV end-diastolic volume (LVEDV).
a. Estimating preload. Unless TEE is in use, LV filling volumes cannot be measured directly. Instead, LVEDV is estimated from a filling pressure (PA diastolic, PCWP, or LA pressure [LAP]). The relationship of LVEDV to LAP and PCWP can be quite variable after bypass secondary to changes in diastolic compliance. Decreased compliance is caused by myocardial edema and ischemia. Therefore, the PCWP is a relatively poor indicator of LVEDV in the post-CPB period.
b. Optimal preload is the lowest value that provides an adequate CO. Preload greater than the optimal value may cause:
(1) Ventricular distention and increased wall tension (increased myocardial oxygen consumption [MVO2])
(2) Decreased coronary perfusion pressure
(3) Excessive or decreased CO
(4) Pulmonary edema
c. Typical weaning filling pressures. For patients with good LV function preoperatively, PCWP of 8 to 12 mm Hg or CVP of 6 to 12 mm Hg often suffices. Abnormal contractility or diastolic stiffness may necessitate much higher filling pressures to achieve adequate filling volumes (20 mm Hg or higher), but in such cases it is imperative to monitor left heart filling by a PA or LA line, or by TEE.
d. CVP/LAP ratio. Normally, the CVP is equal or lower than the LAP, which is usually estimated by the PA diastolic pressure (CVP/LAP ratio less than or equal to 1). If the ratio is elevated (greater than 1), the intraventricular septum may be forced toward the left, limiting LV filling and CO. This “septal shift” often can be diagnosed by TEE as well. In this situation, termination of CPB may be impossible until the ratio is normalized by improving RV function [14].
B. Step 2: Lowering pump flow into the aorta
1. Attaining partial bypass. The rise in preload causes the heart to begin to contribute to the CO. This condition is termed partial bypass because the venous blood draining into the right atrium divides into two paths: Some goes to the pump, and some passes through the RV and lungs and is ejected into the aorta by the LV.
a. Some institutions advocate keeping the patient on partial CPB for several minutes to wash vasoactive substances from the lungs before terminating CPB.
2. Reduced pump outflow requirement. Because two sources of blood are now supplying the aorta, the amount of arterial blood returned from the pump to the patient can be reduced as native CO increases to maintain total aortic blood flow. Therefore, the perfusionist lowers the pump flow rate in increments of 0.5 to 1 L/min. This step is repeated, allowing gradual reductions in pump flow rate while cardiac function and hemodynamics are carefully monitored.
3. Readjusting venous line resistance. Some adjustment in the venous line resistance may be needed to maintain a constant filling pressure as the heart is given more work to perform. Also, as arterial pump outflow is reduced, less venous inflow is needed to keep the venous reservoir from being pumped dry. Therefore, the venous line clamp can be progressively tightened to achieve the desired increase in preload.
C. Step 3: Terminating bypass. If the heart is generating an adequate systolic pressure (typically 90 to 100 mm Hg for an adult) at an acceptable preload with pump flows of 1 L/min or less, the patient is ready for a trial without CPB, and bypass is terminated. The pump is stopped and the venous cannula is clamped. If hemodynamics are not satisfactory, CPB is reinstituted, and management of cardiovascular decompensation is begun (see Section IV.D. below).
IV. Sequence of events immediately after terminating CPB
A. Preload: Infusing blood from the pump. If cardiac performance is inadequate, small increases in preload may be beneficial. For adult patients, volume is transferred in 50- to 100-mL increments from the venous pump reservoir to the patient through the aortic cannula. Before volume infusion, the aortic cannula should be inspected for air bubbles within its lumen. Increments of 10 to 50 mL are used in pediatric patients. During volume infusions from the pump, the BP, filling pressure, and heart should be watched closely. Continuous infusion is contraindicated because overdistention of the heart may occur and the oxygenator reservoir may be emptied, infusing air into the patient.
1. The almost instantaneous infusion of volume by the pump allows for evaluation of LV function. You can assume that during the infusion there is no change in SVR. According to the formula:
BP = CO × SVR
If you make SVR a constant, then:
BP = CO
An increase in BP with a small volume infusion must indicate an increase in CO.
2. If BP and CO do not change with increased preload, the patient probably is at the top (flat part) of the Frank–Starling curve, and further volume infusion is unlikely to be of benefit.
3. If BP does rise, the rise is probably due to a rise in CO, and further volume administration may be beneficial. In this manner, the optimal preload can be titrated after CPB. The TEE can be helpful here.
4. Three factors often contribute to a need to give volume after CPB:
a. Continued rewarming of peripheral vascular beds results in vasodilation.
b. Changes in LV diastolic compliance alter optimal filling pressure.
c. Continued bleeding.
B. Measuring cardiac function
1. Before taking the relatively irrevocable steps of removing the aortic cannula or administering protamine, cardiac function should be assessed because an adequate BP may be the result of a low CO and a high SVR. Cardiac function may be assessed by measuring CO or by TEE. The derived cardiac index (CO/body surface area) should be calculated. Generally, a cardiac index of more than 2 L/min/m2 should be present to consider permanent termination of CPB, although an index of greater than 2.2 usually is considered “normal.” If HR is high, a normal CO can exist despite a low stroke volume. Therefore, a calculation of the stroke volume index (cardiac index/HR) can be useful (normal is greater than 40 mL/beat/m2).
2. Measuring patient perfusion. Signs of adequate tissue perfusion after CPB should be sought. Within the first 5 to 10 min after terminating CPB, arterial blood gases and pH should be measured, looking for lactic acidosis or gas exchange abnormalities. Mixed venous oxygen saturation (SVO2) indicates global body O2 supply–demand balance. Urine output indicates adequacy of renal perfusion and normally rises after CPB, and lack of such a rise should be evaluated and treated immediately. The ideal perfusion pressure for adequate tissue perfusion should be individualized. Patients with renal insufficiency, cerebrovascular disease, or hypertension may require higher perfusion pressures, although the increased BP may worsen bleeding.
3. Afterload and aortic impedance. In the presence of good LV function (and the absence of myocardial ischemia), the anesthesiologist should avoid elevated afterload (as reflected by systolic BP) to prevent excessive stress on the aortic suture lines and to reduce surgical bleeding. In adults, the usual desired range for systolic BP is 100 to 130 mm Hg.
With impaired LV function or valvular regurgitation, SVR should be reduced to the lowest level possible while maintaining adequate BP for organ perfusion. Reducing the aortic impedance improves LV ejection and lowers systolic LV wall stress and myocardial O2 demand. Impedance is related to BP and SVR, and lowering SVR can result in increased CO with no change in BP.
C. Removing the cannulas
1. Venous cannula(s). The presence of a large cannula(s) in the right atrium or in the vena cava will impair venous return to the heart, and if cardiac function is reasonable, the venous cannula should be removed as soon as practical. Removing the cannula will allow the perfusionist to “reprime” the pump and allow for further volume infusion through the aortic cannula.
2. Aortic cannula. Removal of the aortic cannula should usually wait until at least half of the protamine dose has been infused and cardiovascular stability confirmed.
D. Cardiovascular decompensation
1. Refer to Chapter 2 for specific drug pharmacology and doses.
2. Failure of the LV or RV, both of which are recovering from the insult of CPB, together with low SVR are the most common causes of cardiovascular insufficiency during the weaning process.
a. LV failure
(1) The differential diagnosis of LV failure after CPB is listed in Table 9.1.
Table 9.1 Differential diagnosis of LV failure after CPB


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








