Granulopoiesis, Marrow Release, and Margination
The bone marrow is a large organ (70% larger than the liver) and 50%-60% of its function is dedicated to producing neutrophils. Approximately 100 billion neutrophils leave the bone marrow each day in a healthy adult. The normal ratio of neutrophils to erythroid cells ranges from 2:1 to 3:1. As cells differentiate from hematopoietic stem cells, they undergo
five divisions, from myeloblast to promyelocyte to myelocyte. After the myelocyte stage, they no longer undergo meiosis but remain in the large storage pool (
Fig. 83.1). They mature in the storage pool for ˜5 days under normal conditions. The nucleus contracts from the large, ovoid shape of the promyelocyte to the “band,” and finally to the mature neutrophil with its three- to five-lobed nucleus. Neutrophils circulate for up to 12 hours after their release into the bloodstream.
The elimination of foreign microorganisms through phagocytosis, generation of reactive oxygen metabolites, and release of microbicidal substances is dependent on the mobilization

of neutrophilic granules and secretory vesicles. The mature neutrophil contains four granule populations. These granules share common structural features, such as a phospholipid bilayer and an intragranular matrix that contains proteins destined for exocytosis or delivery to the phagosome. Proteins synthesized at the same stage of myeloid cell development localize to the same granule (
3). The primary, or azurophil, granule forms during the myeloblast and promyelocyte stage and contains myeloperoxidase and neutrophil elastase (
Fig. 83.1 and
table 83.1). Production of myeloperoxidase ceases at the promyelocyte-to-myelocyte stage. Secondary (specific)
granules, which are found in myelocytes and metamyelocytes, contain high concentrations of lactoferrin and low concentrations of gelatinase (matrix metalloproteinase 9, MMP-9). The tertiary granules (gelatinase), found in band cells and segmented neutrophils, are low in lactoferrin and high in gelatinase. The secretory vesicles form in segmented neutrophils. Their membranes contain cytochrome
b558 (one of the components of the NADPH oxidase system), receptors for complement [CD35 (complement receptor 1, CR1), CD11b/CD18 (Mac-1, CR3)], receptor for complement component 1q (C1qR), and receptors for monovalent and polyvalent immunoglobulin (CD32, CD64, CD16) and for bacterial lipopolysaccharide (CD14). Exocytosis of granules occurs in reverse order, with secretory granules being the easiest to mobilize and the primary granule the least easy, requiring strong phagocytic stimuli.
The primary granule (
table 83.1) contains a number of antimicrobial peptides: the four
α-defensins (human neutrophil peptide 1 through 4), bactericidal/permeability-increasing protein (
BPI), and serprocidins (serine proteases with microbicidal activity: proteinase-3, cathepsin G, and elastase). The
α-defensins have microbicidal activity against a broad range of fungi, bacteria, enveloped viruses, and protozoa. They exert their effect through the formation of transmembrane pores. Neutrophil defensins induce chemotaxis of monocytes and lymphocytes.
BPI is highly cationic, kills Gram-negative bacteria at nanomolar concentrations, and neutralizes lipopolysaccharide. The serprocidins are cationic polypeptides with proteolytic activity against a variety of extracellular matrix proteins, such as elastin, fibronectin, laminin, type
IV collagen, and vitronectin. Unrestrained release of elastase plays a crucial role in the pathogenesis of pulmonary emphysema. The serine proteases have a number of inhibitors: those found in the systemic circulation and in the primary granules (
α1-antitrypsin, also known as
α1-proteinase inhibitor, and
α2-macroglobulin) and those produced by the epithelial and immune cells (secretory leukoproteinase inhibitor and elafin).
The specific and gelatinase granules are formed in the meta-myelocyte stage and continue through maturation until the formation of segmented neutrophils. Specific granules are larger and rich in antibiotic substances. Being more difficult to mobilize than gelatinase granules, they release their contents into the phagolysosome or the exterior of the cell. Gelatinase granules are smaller and more easily exocytosed. They are important primarily as a reservoir of matrix-degrading enzymes and membrane receptors required during neutrophil extravasation. Neutrophils contain three metalloproteinases (MMPs): neutrophil collagenase (MMP-8), gelatinase (MMP-9), and leukolysin (MMP-25) (table 83.1). The MMPs are able to degrade major structural components of the extracellular matrix and are important for the extravasation of neutrophils.
The secretory vesicles are endocytic and constitute a reservoir of membrane-associated receptors required at the earliest phases of neutrophil localization. The secretory vesicles are mobilized in response to a wide variety of inflammatory stimuli. The membranes are rich in CD11b/CD18 (Mac-1, CR3), CD35 (CR1), and receptors for the formylated bacterial peptide [formylmethionyl-leucyl-phenylalanine (fMLF), CD14, and CD16 (FcγRIIIb receptor]).
Maturation of the neutrophil and granule protein synthesis are achieved by the sequential and combined action of transcription factors. More than 40 different growth factors, cytokines, and chemokines regulate
PMNL proliferation, differentiation, and cell fate. The most clinically well-known is granulocyte colony-stimulating factor (G-CSF). G-CSF specifically promotes neutrophil proliferation and maturation and enhances neutrophil microbicidal activity when administered in vivo. G-CSF interacts with relatively late hematopoietic progenitors that have already committed to the neutrophil lineage and serves to support their growth and final maturation into functional neutrophils. Granulocyte-macrophage colonystimulating factor (
GM-CSF) acts on progenitors that are committed to produce either neutrophils or monocytes but can also act on granulocyte precursors directly. As with G-CSF, GMCSF can enhance neutrophil reactivity when given in vivo.
Neutrophils continuously egress from the sinusoids of the bone marrow. Release is regulated by chemokine receptors expressed on the cells, and their ligand chemokines expressed by stromal cells. Within the circulation, about half of the neutrophils are in the flowing stream; the other half are inaccessible to phlebotomy. This half is called the
marginating pool. In response to stress, exercise, or
IV epinephrine, the neutrophils in the marginating pool are released into the circulating pool. The marginating pool of neutrophils is in the postcapillary venules in major organs and in the capillaries of the lungs. As a result of longer transit times of neutrophils compared with erythrocytes in normal lungs, the concentration of neutrophils within the pulmonary capillary blood is ˜40-80 times higher than it is within the blood in large vessels. Neutrophils travel in “hops” through the lung microvasculature, moving quickly through larger capillary segments but stopping and deforming for entry into smaller segments (hence the longer transit times). The neutrophils marginated in the pulmonary bed differ from mature neutrophils, and have increased CXC chemokine receptor 4 (CXCR4). As CXCR4 increases on neutrophils as they age, and the pulmonary vascular endothelial cells express CXC chemokine ligand 12 (CXCL12; stromal derived stem cell factor 1,
SDF-1), it is possible that “aging” neutrophils populate the pulmonary reservoir (
4).
Neutrophilia occurs after the administration of glucocorticoids. Approximately 60% of the neutrophilia is due to mobilization from the marginated pool, 10% is due to increased bone marrow release, and 30% is due to lengthened half-life in circulation. The administration of G-CSF shortens the transit time of neutrophils through the marrow, particularly in the postmitotic pool. G-CSF has no effect on demargination but delays clearance of neutrophils by inhibiting apoptosis.
In response to inflammatory stimuli or infection, neutrophil production and release significantly increase. Neutrophil release from the bone marrow initially exceeds production, causing a temporary decrease in the bone marrow neutrophil pool. Once the bone marrow neutrophil pool is reestablished, release from the marrow increases and neutrophil count rises. In those individuals with limited neutrophil pool reserves (those with drug-induced neutropenia, those who have received chemotherapy, and in infants) neutrophil count may remain low while the neutrophil pool replenishes.
“Mature” neutrophils released from the bone marrow have altered function after infection, compared with cells produced

during the “noninfected” state. They demonstrate decreased chemotaxis, decreased phagocytosis, and an impaired ability to upregulate CD10, a neutral endopeptidase that is present on only mature granulocytes (
5,
6).
Neutrophil localization in infection
The body has a highly coordinated and regulated response to microbes. The first defense is local immunity. The epithelial surface itself functions as a physical barrier. Antimicrobial peptides are released from the epithelium, and secretory IgA is released from submucosal plasma cells. Microbes may be eliminated or controlled at the source; however, if the microbial burden exceeds these processes, then neutrophil recruitment is required to control the infection. Epithelial-bacterial interactions result in the release of cytokines, specifically
IL-1 and tumor necrosis factor (
TNF)-
α, and chemokines, such as IL-8, CXCL8, and G-CSF (
7). These cytokines activate the macrophages that reside in submucosa, which in turn amplify
the proinflammatory signal with additional release of proinflammatory chemokines and cytokines.
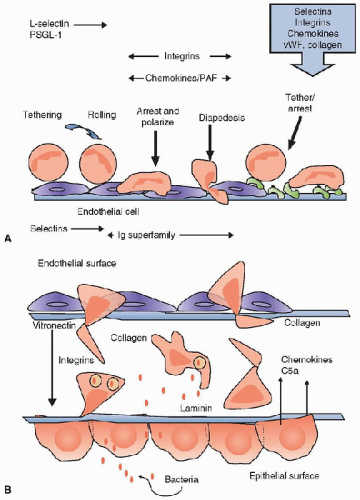
The endothelium of the nearby postcapillary venule, under the immunologic pressure of proinflammatory cytokines, transforms from a nonadhesive surface to one that is proadhesive through the expression of specific ligands on the endothelial surface (
table 83.2). Selectins are responsible for the initial capture of the neutrophil from the free-flowing stream and their rolling on the endothelial surface. Selectins are present on both neutrophils (L-selectin, CD62-L) and the endothelial surface (E- and P-selectin, CD62-E and CD62P, respectively) (
table 83.2) (
8). Members of the immunoglobulin superfamily (IgSF), intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule (
VCAM) are critical for neutrophil slowing, arrest, and migration on the cell surface (
Fig. 83.2). The receptors on the neutrophil surface for the IgSF are the
β2 integrins: Mac-1 (CD11b/CD18, CR3), leukocyte functional antigen 1 (LFA-1) (CD11a/CD18), and the
β1-integrin VLA-4 (CD49d/CD29) (
table 83.2). The leukocyte integrins are heterodimers comprising
α and
β subunits. The
β subunit may be shared by multiple members of a subfamily, whereas the
α subunit confers specificity. The leukocyte integrins are generally functional in an “inactive state” (
9). Through a process known as
inside-out signaling, the integrins change their conformation and become “active,” and ligand recognition can occur. The endothelial surface secretes a number of
chemokines and lipid-derived products, such as CXCL8 (IL-8) and platelet-activating factor (
PAF), which can activate the neutrophil through specific receptors (
table 83.3). This step is critical for mediating the transition from rolling to arrest. Once the leukocyte has arrested, it polarizes then “crawls” (also known as diapedesis) through the endothelial lining of the vessel, either between endothelial cells (transendothelial migration, TEM) or less commonly across the endothelial cell itself (transcellular migration) (
10). Most leukocyte TEM occurs in postcapillary venules. Movement of the neutrophil across the endothelium is dependent on a sequential engagement of adhesion molecules. On the apical surface of the
endothelium, ICAM-1 and VCAM-1 function in the activation and adherence of captured leukocytes. In the intercellular borders,
PECAM and CD99 are required for diapedesis in vitro. In vivo, other molecules are involved, including JAM-A and ICAM-2. Recent work suggests that the site of TEM is the LBRC (
10). While movement across the endothelium is rapid, the neutrophils must then breach the venular walls, including the pericyte sheath and the venular basement membrane. This requires the pericyte to express key adhesion molecules (e.g., ICAM-1 and VCAM-1) and chemokines (
8). Perivascular mast cells and macrophages are also rich sources of neutrophil and monocyte chemoattractants. The egress across the venular wall is significantly slower than across the endothelium itself.
 Neutrophils are uniquely designed to deal with the host’s interaction with microbes at mucosal and epithelial surfaces.
Neutrophils are uniquely designed to deal with the host’s interaction with microbes at mucosal and epithelial surfaces. Neutrophils released from the bone marrow during times of acute infection and injury function differently compared with those released during periods of normal hematopoiesis.
Neutrophils released from the bone marrow during times of acute infection and injury function differently compared with those released during periods of normal hematopoiesis. Transfusion-related acute lung injury is more common than previously thought and should be considered in any patient with acute deterioration in lung injury. The effector cell is the neutrophil.
Transfusion-related acute lung injury is more common than previously thought and should be considered in any patient with acute deterioration in lung injury. The effector cell is the neutrophil. The use of granulocyte colony-stimulating factor (G-CSF) decreases hospitalization time of neutropenic patients, whereas its use in the ICU population is far from clear. G-CSF use and recovery from neutropenia may result in lung injury.
The use of granulocyte colony-stimulating factor (G-CSF) decreases hospitalization time of neutropenic patients, whereas its use in the ICU population is far from clear. G-CSF use and recovery from neutropenia may result in lung injury. Granulocyte transfusion and the transfusion of activated neutrophils with blood cell transfusion may contribute to increased organ dysfunction, particularly pulmonary dysfunction. Leukocyte depletion of blood prior to use in extracorporeal membrane oxygenation circuits or use of leukocyte depletion filters in patients in cardiopulmonary bypass may be of use.
Granulocyte transfusion and the transfusion of activated neutrophils with blood cell transfusion may contribute to increased organ dysfunction, particularly pulmonary dysfunction. Leukocyte depletion of blood prior to use in extracorporeal membrane oxygenation circuits or use of leukocyte depletion filters in patients in cardiopulmonary bypass may be of use. of neutrophilic granules and secretory vesicles. The mature neutrophil contains four granule populations. These granules share common structural features, such as a phospholipid bilayer and an intragranular matrix that contains proteins destined for exocytosis or delivery to the phagosome. Proteins synthesized at the same stage of myeloid cell development localize to the same granule (3). The primary, or azurophil, granule forms during the myeloblast and promyelocyte stage and contains myeloperoxidase and neutrophil elastase (Fig. 83.1 and table 83.1). Production of myeloperoxidase ceases at the promyelocyte-to-myelocyte stage. Secondary (specific)
of neutrophilic granules and secretory vesicles. The mature neutrophil contains four granule populations. These granules share common structural features, such as a phospholipid bilayer and an intragranular matrix that contains proteins destined for exocytosis or delivery to the phagosome. Proteins synthesized at the same stage of myeloid cell development localize to the same granule (3). The primary, or azurophil, granule forms during the myeloblast and promyelocyte stage and contains myeloperoxidase and neutrophil elastase (Fig. 83.1 and table 83.1). Production of myeloperoxidase ceases at the promyelocyte-to-myelocyte stage. Secondary (specific)  during the “noninfected” state. They demonstrate decreased chemotaxis, decreased phagocytosis, and an impaired ability to upregulate CD10, a neutral endopeptidase that is present on only mature granulocytes (5,6).
during the “noninfected” state. They demonstrate decreased chemotaxis, decreased phagocytosis, and an impaired ability to upregulate CD10, a neutral endopeptidase that is present on only mature granulocytes (5,6).