Fig. 19.1
Flow diagram representing the 3-staged approach to abdominal closure. Reproduced with permission from Dicocco et al. [7]
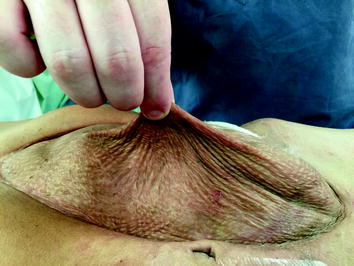
Fig. 19.2
Pinching the split-thickness skin graft from the underlying viscera, indicating the appropriate time for definitive reconstruction
Lessons Learned from Previous Studies
We would like to share some practical lessons that we have learned after studying abdominal wall reconstruction for the past 25 years. In our initial years of planned ventral hernia management, the choice of temporary abdominal closure prosthetic was a decision that was left to the operating surgeon, and the prosthetics included polypropylene mesh, woven vicryl mesh, expanded PTFE soft tissue patch, and plastic (intravenous fluid bag or X-ray cassette cover). The application of a variety of prosthetic materials in our institution in the past has provided a perspective on merits and drawbacks of each material. The removal of polypropylene mesh is not a simple procedure and is very time consuming and carries a higher risk of injury to bowel. The cost and difficulty of removal of expanded PTFE were made evident in the past studies, and the lack of durability of plastic material used for coverage has led to a change in our practice. These factors have been responsible for the gradual switch to absorbable mesh with woven vicryl being chosen most frequently at our institution [5].
Another lesson that we have learned over the past decade is that it is best to immediately place the skin graft at the time of vicryl mesh removal. In our early experience, we performed dressing changes for a couple of days to diminish nosocomial bacterial colonization of the granulated wounds before applying the skin graft. However, we had patients during those few days that eviscerated and had resulting derserosalization that resulted in difficult to management enteric fistula [2]. This has led to our change in practice, and now all patients receive immediate STSG at the time of mesh removal.
We have also determined that it is best to remove the mesh and place the skin graft no later than 3 weeks after mesh placement [2]. Those patients who had mesh retention beyond 3 weeks were found to have a significant increased risk of intestinal fistula. For this reason, we strive to remove the vicryl mesh and place STSG on these patients approximately 2 weeks after placement of the vicryl mesh.
There have also been many proponents of vacuum-assisted closure (VAC) to aid in the delayed primary fascial closure rates. We performed a prospective randomized trial to address the rates of delayed fasical closure and fistula rates comparing temporary closure with vicryl mesh versus VAC in patients with open abdomens [6]. Our results indicated that the VAC group trended toward increased morbidity. There was an increase in fistula rates in the VAC group, and we believe this was due to the need for repeated vacuum changes that are not required with vicryl mesh. We also found that enteral gastrostomy and jejunostomy tubes that were placed had a higher rate of becoming dislodged with the repeated vacuum dressing changes. We also had patients with loss of domain and massive visceral edema that experienced evisceration with VAC closure. The study found that neither method was superior in regard to the rates of delayed fascial closure. For these reasons, we have adopted absorbable vicryl mesh followed by skin grafting for those patients in whom delayed fascial closure seems unlikely.
We have also found that delaying definitive abdominal wall reconstruction beyond 1 year directly contributes to the need for prosthetic mesh as well as increases the rate of hernia recurrence [2]. Delay beyond 1 year leads to loss of domain and decreased fascial compliance due to contraction and consequent retraction of the abdominal wall fascia laterally. For this reason, we carefully follow all patients discharged with planned ventral hernias at 3-month intervals to assess the readiness of removal of their skin graft and definitive reconstruction.
Review of Abdominal Wall Anatomy
Published in 1990 by Ramirez et al. [1], component separation is based on subcutaneous lateral dissection, fasciotomy lateral to the rectus abdominis muscle, and dissection on the plane between external and internal oblique muscles with the medial advancement of the block that includes the rectus muscle and fascia. This release allows 3–5 cm of the additional length on each side. Because of the giant abdominal wall defects (Fig. 19.3) that occur after open abdomens, we developed a modification that results in an additional 20 cm in the umbilical region that allows the closure of many giant planned ventral hernias with native tissue only.
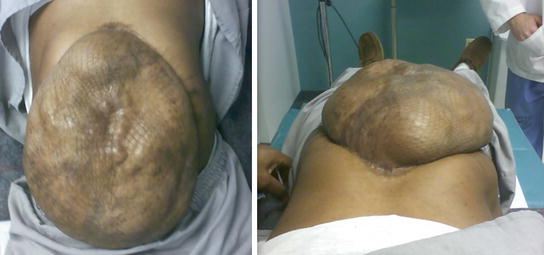

Fig. 19.3
Preoperative images of a patient with a planned ventral hernia. Giant abdominal wall defect is covered with skin graft over the abdominal viscera. Reproduced with permission from Dicocco et al. [7]
Before describing the technique in depth, a brief review of the abdominal wall anatomy (Fig. 19.4) will facilitate understanding the procedure. The rectus muscle is surrounded by the anterior and posterior rectus sheaths. The external is a component of the anterior rectus sheath for the entire length of the rectus. The internal oblique splits and contributes to both the anterior sheath and posterior sheath above the arcuate line. However, below the arcuate line, the entirety of the internal oblique joins the anterior sheath. The transversus abdominis also contributes to the posterior sheath above the level of the arcuate line, but likewise joins the anterior sheath inferior to this landmark. This leaves no posterior sheath below the arcuate line and where only the peritoneum is present.
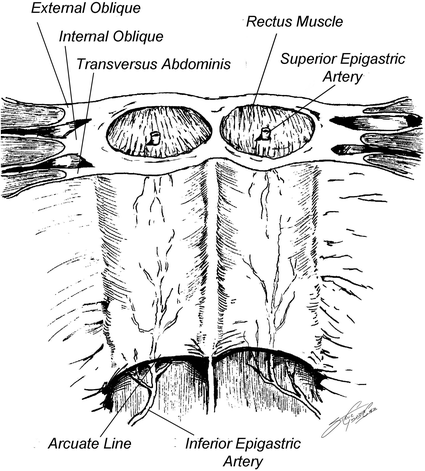

Fig. 19.4
Diagram of abdominal wall anatomy, shown from the intraperitoneal surface, with cut edge representing the cephalad portion. The arcuate line, vascular supply, and components of the rectus fascia can be seen. Reproduced with permission from Dicocco et al. [7]
The blood supply to the rectus is supplied by the superior epigastric and the deep inferior epigastric arteries, with the inferior providing the major component. The inferior epigastric artery lies between the internal oblique and transversus abdominis muscles. It enters the rectus sheath around the arcuate line. Therefore, separation of the anterior rectus sheath laterally does not compromise blood supply. Blood supply to the anterior sheath is also from epigastric vessels. For this reason, separation of the anterior sheath from the rectus is not ideal. The posterior sheath obtains its vasculature from the vessels supplying the peritoneum and can be separated from the rectus without becoming devascularized.
Preoperative Evaluation in Preparation for Planned Ventral Hernia Repair
Patients that are managed with an open abdomen and a staged management algorithm often have a prolonged hospital course. Upon discharge, we closely follow these patients in clinic. Once out of the acute phase, we see these patients at 3-month intervals to assess the readiness of the skin graft for removal. The technique, as mentioned before and seen in Fig. 19.3, requires lifting and “pinching” the graft to see whether it can be lifted off the underlying viscera. The technique takes some experiences, and the decision to proceed with definitive abdominal wall reconstruction is made by the attending physician. In preparation for reconstruction, many of these patients also have diverting ostomies that require endoscopy and/or contrasted enemas to determine whether they are ready to be reversed. These studies are obtained concurrently with the preparation for their planned ventral hernia repair. Because we use the modification of the component separation at our institution frequently, if we anticipate the patient will have a planned ventral hernia, the initial ostomy location can be placed lateral to the rectus sheath so that the fascial defect from the ostomy can be closed primarily or with a small piece of biologic mesh. Again, we strive to repair all planned ventral hernias between 8 and 12 months after the placement of the skin graft to avoid the loss of domain that comes if the operation is delayed for more than 1 year.
Preoperative assessment can be deceiving, so the reconstruction technique should be decided intraoperatively. Patients who have very large defects may have mobile fascia and can be closed with a standard component separation. Conversely, some patients with small defects may require the Memphis modification and possible addition of prosthetic material.
This patient population has had significant physical and psychological stress due to their major trauma and prolonged hospital course. We have long discussions with these patients before proceeding with definitive reconstruction. They are again faced with a 7- to 10-day hospital stay, if no complications arise. We review in-depth complications that can occur and make patients aware that abdominal wall reconstruction results in a significant abdominal pressure and transient discomfort postoperatively. We often use epidural catheters perioperatively in these patients to help with pain control.
Operative Technique of Memphis Modification for Component Separation
Now we would like to provide a step-by-step description of our technique and include some intraoperative pearls and pitfalls that we have learned [7].
- 1.
Removal of the skin graft. Beginning in an area that is loosely adherent, there will be areas with dense adhesions. In these areas, a plane between the dermis and epidermis is developed. Small areas of skin graft can be left on the viscera to avoid serosal injuries without any consequence. The skin graft is excised from the native abdominal wall laterally to preserve skin edges. Once the graft is removed, the bowel is examined thoroughly for serosal injuries or enterotomies, which are not uncommon. Ostomy reversal is done at this point.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








