Chapter 3 The physiology of pain
Introduction
The International Association for the Study of Pain defines pain as ‘an unpleasant sensory or emotional experience associated with actual or potential tissue damage or described in terms of such damage’ (Merskey 1979). It is an experience influenced by sensory, affective, motivational and sociocultural elements, with many factors apart from the intensity of the noxious stimulus determining the way in which it is perceived (Shipton 1999). Pain is always subjective (Prologue). Although it is an unpleasant experience, the absence of pain is far from blissful. Individuals with congenital absence of pain either due to failure of nociceptor survival in the embryo or channelopathies have congenital pain hyposensitivity leading to damaged limbs and joints and early death which highlights the importance of nociception as a protective mechanism.
The pain perception threshold is the threshold at which a subject perceives pain. Interestingly this shows little interindividual variability. For a thermal stimulus, this is around 44–45°C. However, the pain tolerance threshold, the maximum amount of pain that a person is able to tolerate, differs widely (Baldry 2001). Pain is greatly affected by the context in which the pain is experienced. For example, soldiers sustaining severe battlefield injuries often feel little pain at the time, while the pain of fibromyalgia, with little evidence of tissue damage can lead to an individual functioning at a very low level (Chapter 5). Pain caused by tissue damage cannot be differentiated from that without tissue damage (Merskey 1979).
The nervous system is highly adaptive. The variable and non-linear nature of these properties means adaptation of the nervous system at an individual level is inherently unpredictable and, like any complex adaptive system, can only be observed after the fact, rather than predicted (Plsek & Greenhalgh 2001).
A deeper understanding of pain has paralleled the understanding of the nervous system. In 1664, the philosopher and scientist Rene Descartes described in his ‘Treatise of Man’ the transmission of pain through a single hardwired channel from the skin to the brain. Its stimulation led to involuntary withdrawal of a foot on application of a noxious stimulus. Signals transmitted along the pain pathway are in fact subject to modulation at different levels. Rather than being hardwired as proposed by Descartes, neurological pathways are in fact plastic, reorganizing and learning from previous experience. This plasticity remains possible even in adult life (Pearce & Merletti 2006). The brain and spinal cord learn to facilitate activity in commonly utilized pathways. Such changes occur in relation to useful information such as practical tasks, innocuous details as well as unpleasant information such as pain (Deleo 2006, Holdcroft 2005).
Classification of pain
Terminology
Hyperalgesia and allodynia
Common signs are hyperalgesia and allodynia. Hyperalgesia is an exaggerated pain response to a noxious stimulus, while allodynia is a pain response produced by a non-noxious stimulus. Sensitization of the peripheral and central nervous system are the underlying mechanisms for these signs (Table 3.1).
Table 3.1 A mechanism-based classification system for pain (Woolf 1998)
| Pain Category | Possible Primary Afferent Mechanism | Possible Central Mechanism |
|---|---|---|
| Transient pain (response to noxious stimulus with no long-term effect e.g. pin prick) | Nociceptor specialization | — |
| Tissue injury pain | Sensitization, recruitment of silent nociceptors, alteration in phenotype, hyperinnervation | Central sensitization and recruitment, summation, amplification |
| Nervous system injury pain | Acquisition of spontaneous and stimulus evoked activity by nociceptor axons at loci other than peripheral terminals, phenotype change | Central sensitization, deafferentation of second-order neurons, disinhibition and structural reorganization |
Nociceptive and neuropathic pain
Nociceptive pain occurs upon stimulation of nociceptors by mechanical, thermal or chemical stimuli (Woolf 1991). Neuropathic pain occurs as a result of damage to the peripheral or central nervous system rather than stimulation of nociceptors and can continue even after cessation of the original stimulus (Woolf 1991).
Acute versus chronic
Acute pain resolves within hours, days or weeks and is caused by trauma or inflammation. Pain over more than three months is artificially defined as chronic. The boundary blurs between when an acute pain becomes chronic, but pain persisting beyond what is ‘normal’ for a certain injury may also be considered chronic (Breivik et al 2006). Spontaneous pain is a common symptom in chronically painful conditions (Woolf 1995).
Pain in the evolutionary context
The primary function of pain can be understood from the evolutionary perspective. Acute pain serves as a warning system and alerts the organism to tissue damage or injury that needs to be addressed. Nociceptive pain has an essentially protective role as it results in a flexion withdrawal response. The CNS allows learning to avoid similar potentially tissue-damaging stimuli in future (Woolf 1989).
Somatic pain is protective, encouraging the person to escape from the noxious stimulus. The natural response of immobility prevents further tissue damage. Hypersensitivity in the injured area prevents contact with other external stimuli, while hypervigilance causes a range of protective behaviours to prevent further damage (e.g. licking, rubbing, looking and holding) (Woolf 1995).
Neuroanatomy: the pain network
The peripheral nervous system
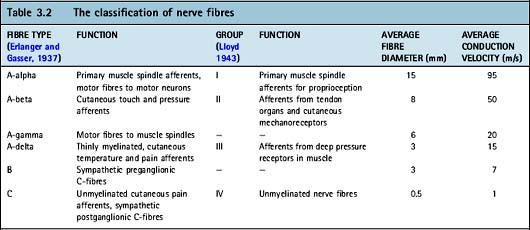
Primary sensory neurons have cell bodies outside of the spinal cord within the dorsal root ganglion. A receptor at the end of each neuron lies in the peripheral tissue and transduces environmental signals into an action potential (see later). This is transmitted along an axon which terminates in the dorsal horn of the spinal grey matter (Table 3.2). Sensory neurons can be classified according to cell body size, axon diameter, conduction velocity, degree of myelination and response to various neurotrophic factors.
A-fibres have large cell body diameters and are divided into three subgroups: A-alpha, A-beta and A-delta fibres. A-alpha fibres innervate muscle spindles and golgi tendon organs and their main function is proprioception (Chapter 6.4). A-beta fibres are low-threshold, cutaneous mechanoreceptors.
Nociceptive neurons are divided into A-delta fibres (20%) and C-fibres (80%). A-delta fibres tend to be associated with mechanical and thermal nociceptors (Holdcroft 2005) and respond to one stimulus only (unimodal). Stimulation of A-delta fibres results in sharp, well-localized pain felt almost immediately (Davies & Blakeley 2001). C-fibres have small diameter, unmyelinated axons with free nerve endings (Rang et al 1991, Woolf 1991) associated with polymodal nociceptors which can be stimulated by thermal, mechanical or chemical stimuli. C-fibres cause slower, poorly localized pain. Both C and A-delta fibres may be associated with ‘silent nociceptors’. These have very high thresholds for mechanical stimulation and do not fire under normal circumstances (Meyer et al 2006). Nociceptive neurons are found in somatic tissues such as skin, joints, muscle, fascia, tendons, cornea and tooth pulp (Cimino 1992). The concentration of nociceptive neurons within a tissue is positively correlated to that tissue’s sensitivity to pain. Nociceptive neurons are also found in viscera, but there are relatively fewer A-delta fibres.
Nociceptive neurons are further divided into two major subgroups: those expressing peptides (peptidergic) and those that do not (non-peptidergic). The majority of peptidergic neurons co-localize substance P (SP) and calcitonin gene-related peptide (CGRP) whilst others contain vasoactive intestinal peptide (VIP) and somatostatin (SS) (Woolf & Ma 2007). Peptidergic nociceptive neurons also contain excitatory neurotransmitters (glutamate). Peptidergic C-fibres project mainly to lamina I and lamina II outer layers of the dorsal horn. Non-peptidergic neurons project to areas within lamina II and contain only neurotransmitters.
Peptidergic and non-peptidergic fibres have different processes of signal transduction and demonstrate different sensitivities to the same stimulus (McMahon et al 2006). Nociceptors are dynamic and shift their properties according to their environment (Schmidt et al 1994). This plasticity allows mediation of homeostatic functions under physiological conditions and sensitization following injury or inflammation.
Nociceptive neurons develop from neural crest stem cells that migrate from the dorsal part of the neural tube and are formed late in neurogenesis under the neurotrophic influence of nerve growth factor (NGF) interacting with the tyrosine kinase A (trkA) receptor in the periphery. During perinatal and postnatal development approximately half the nociceptive population switch off the genes necessary for (TrkA) expression. Most of these fibres switch to expressing Ret, the transmembrane signalling component of the receptor for glial cell-derived neurotrophic factor (GDNF) as well as other neurotrophic factors (McMahon et al 2006). These neurons become the non-peptidergic C-fibres and express surface glucoconjugates that bind the lectin IB4 (Molliver et al 1997). Tyrosine kinase activity continues in the remaining half of C-fibres. A-delta fibres express the high-affinity receptor for neurotrophin 3 and tyrosine kinase C. Sensory neurons remain responsive to neurotrophic factors into adulthood and they have effects on the pain experience.
Primary afferent neurons can switch their phenotype as the result of changes in gene expression leading to an up-regulation of various transmitters, receptors, ion channels and growth factors (Table 3.3). After inflammation or injury A-beta fibres can produce and release SP which they do not contain under normal circumstances. This is important for the development of mechanical allodynia which is mediated by A-beta fibres (Coderre et al 2003). Neurotrophins are thought to play an important part in this.
Table 3.3 Molecules up-regulated in phenotypic changes of primary sensory afferents
| Molecule | Example |
|---|---|
| Transmitter | |
| Receptors | |
| Ion channels | |
| Growth factors |
The spinal cord
The cells in the spinal cord are arranged in laminae. The dorsal horn of the grey matter of the spinal cord is conventionally divided into six layers or laminae (I–VI) on the basis of Rexed’s descriptions of their appearance under light microscopy; the ventral horn is divided into three further laminae (laminae VII–IX); and another column of cells arranged around the central canal is lamina X (Cramer & Darby 2005). A-delta and C-fibres carrying nociceptive information terminate in laminae I and II (also known as the substantia gelatinosa because of its translucent appearance on light microscopy).
Primary afferent input to the dorsal horn is mostly ipsilateral (same side), however, there is some contralateral (opposite side) input. Many dorsal horn neurons, especially in lamina II are interneurons which project locally within the spinal cord. Excitatory interneurons contain glutamate, whereas inhibitory interneurons contain GABA, enkephalins and dynorphin. The deep laminae of the dorsal horn contain many pre-motor interneurons and have many projections to ventral horn neurons, the cerebellum and reticular formation where they influence sensorimotor coordination and behaviour (Dostrovsky & Craig 2006).
Microglia and astrocytes are glial cells in the CNS. They are important in modulating neuronal excitability and synaptic transmission and are involved in the initiation and maintenance of neuropathic pain (Hains & Waxman 2006, Hashizume et al 2000).
Projection neurons in the spinal cord transmit information supraspinally and have indirect polysynaptic input from excitatory and inhibitory interneurons allowing processing of information (Coderre et al 2003). Transmission of different types of nociceptive information occurs through two separate pathways, each with its own conduction velocity and termination in the brain (Kerr et al 1955). Phylogenetically, the younger tract is the neospinothalamic tract (STT) while the spinoreticular tract (paleo-spino-reticulo-diencephalic pathway) is older.
The STT is the most prominent ascending nociceptive pathway and arises mainly from laminae I and V of the dorsal horn where most A-delta nociceptive fibres terminate. It passes contralaterally over several spinal cord levels via Lissauer’s tract to ascend to the thalamus. This pathway is arranged topographically and placed laterally (predominantly from lamina I) and anteriorly (predominantly from laminae V and VII) within the spinal cord (Dostrovsky & Craig 2006). It is responsible for the identification and localization of a noxious stimulus and determining when this crosses the pain threshold (Melzack & Casey 1968).
STT fibres terminate in either the medial laminae (lateral spinal tracts) or the lateral laminae of the thalamus (anterior spinal tracts). STT terminations from lamina V neurons tend to occur in the ventroposterior nucleus and in the ventral lateral nucleus (VPL). These lateral tracts project from here to the primary somatosensory cortex (Dostrovsky & Craig 2006) and are responsible for the ‘sensory-affective’ aspects of the pain experience. The posterior part of the ventral medial nucleus of the thalamus (VMpo) serves as a thalamocortical relay nucleus for lamina I STT cells to the insula. Other medial tracts project to the insula and anterior cingulated cortex.
The spinoreticular tract carries noxious information from sensory afferents which terminate in laminae I and II. The tract ascends contralaterally alongside the STT. It separates in the brain and takes a medial route to the brainstem. The tract terminates in four main areas: catecholamine cell groups (ventrolateral medulla, nucleus of the solitary tract, and locus coeruleus) involved in sympathetic outflow; the parabrachial nucleus; the periaqueductal grey matter; and the reticular formation (Dostrovsky & Craig 2006). There are also projections to the anterior cingulate, limbic and frontal cortices. This tract is responsible for the slow, aching, emotional aspects of the pain experience and important for overall cognitive effects (Dostrovsky & Craig 2006).
Some ascending nociceptive information is also transmitted along the spinomesencephalic tract, cervicothalamic tract and spinohypothalamic tract (Basbaum & Jessell 2000). The spinomesencephalic tract arises from laminae I and V neurons and projects to the mesencephalic limbic system and via the parabrachial nuclei to the amygdala and is also involved in the affective component of pain. The cervicothalamic tract arises from the lateral cervical nucleus of the upper two cervical segments of the spinal cord which receives input from nociceptive neurons in laminae III and IV. Most of these axons ascend contralaterally to nuclei in the midbrain and the ventroposterolateral and posteromedial nuclei of the thalamus (Basbaum & Jessell 2000). The spinohypothalamic tract arises from laminae I, V and VIII and projects directly to supraspinal autonomic control centres and activates neuroendocrine responses (Basbaum & Jessell 2000).
Descending pathways
A descending inhibitory system was first discovered by stimulation of the periaqueductal grey area which produced a profound analgesia allowing surgery without any apparent distress in conscious animals (Reynolds 1969). There are three endogenous descending pathways from the brain for inhibiting pain which are mediated by either serotonin (5-hydroxytryptamine), noradrenaline (norepinephrine) or opioids. Associated brain areas influencing these pathways include the frontal lobe, anterior cingulate cortex, insula, amygdala, hypothalamus, nucleus cuneiformis. The descending pathways involve the medulla, especially the rostroventral medulla (RVM) and the nucleus raphe magnus (NRM) (Gebhart 2004).
Descending noradrenergic input to the spinal cord originates from the dorsolateral pons, locus coeruleus and to some extent the parabrachial nuclei and adrenergic neurons of the ventrolateral medulla (Scholz 2005). Axons then descend to the spinal cord.
The projections of the raphe spinal pathway from the nucleus raphe magnus in the medulla use serotonin (5-hydroxytryptamine) as the neurotransmitter (Dubner & Ren 1999). Axons descend in the dorsolateral funiculus of the spinal cord and synapse with stalked enkephalinergic inhibitory interneurons in the dorsal horn (Basbaum & Jessell 1977). There are also SP and inhibitory enkephalinergic connections between the nucleus raphe magnus and the noradrenergic neurons of the dorsolateral pons (Westlund 2005).
Finally, the diffuse noxious inhibitory control system (DNIC) describes the reduction in pain perception in one part of the body by the application of a noxious stimulus to another area and is mediated by opioids. Endogenous opiate-like substances comprise three groups: the enkephalins, endorphins and dynorphins. These occur throughout the body and act upon mu, kappa and delta opioid receptors within and outside the pain pathways. Mu opioid receptors occur extensively in certain parts of the brain including the medial thalamic nuclei, the reticular formation and the limbic system, as well as within the substantia gelatinosa of the dorsal horn. Only a few occur in the ventrobasal thalamus and post central gyrus associated with the neospinothalamic tract. DNIC is possibly mediated by collaterals linking the neospinothalamic pathway to the subnucleus reticularis dorsalis in the medulla (Villaneuva et al 1988). Axons from here descend in the dorsolateral funiculus and exert an inhibitory effect via the opioid system on neurons responsible for transmitting noxious information to the brain (Bernard 1990).
The brain
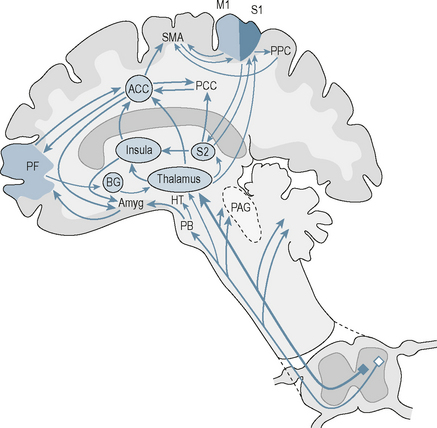
Nociceptive information is transmitted to and processed by a number of brain areas forming a distributed network known as the pain ‘neuromatrix’ (Tracey & Mantyh 2007). On a simplistic level this can be divided into lateral and medial neuroanatomical components with the former having mainly sensory-discriminatory function and the latter having affective and cognitive functions (Tracey & Mantyh 2007) (Fig. 3.1).
The thalamus
All the sensory pathways synapse with thalamic neurons before reaching the cortex. This allows the thalamus to act as the gateway to the cerebral cortex (Tracey & Mantyh 2007).
The cerebral cortex
The outermost neocortex has a characteristic cytoarchitecture with the cell bodies of cortical neurons arranged in six layers parallel to the surface of the brain. Neurons of layer IV receive synaptic input from outside the cortex, mainly from the thalamus, and make short-distance connections with other layers of cortex (Fatterpekar et al 2002). Neurons in the outer layers II and III tend to project to other neurons of the neocortex while those of layers V and VI transmit information out of the cortex to the thalamus, brainstem and spinal cord. The insula cortex is phylogenetically older than neocortex and the anterior and mid-parts are active in human pain experiences. These neurons are dysgranular in microscopic appearance.
The neospinothalamic pathway projects to the primary somatosensory cortex (S1) which receives a large nociceptive input from the ventroposterolateral nucleus of the thalamus (Fatterpekar et al 2002). Like the spinal cord, S1 is arranged somatotopically so that the body homunculus is mapped onto the surface of the cortex with the legs arranged medially and the face laterally. Information is further processed in the secondary somatosensory cortex (S2) and there are close connections with the primary motor cortex as well as parietal inputs.
Limbic system
The main nociceptive inputs to the anterior cingulate cortex (ACC) are from the thalamus (six medial nuclei: midline, intralaminar, central, parafascicular, reuniens and mediodorsal nuclei) and the amygdala. It also links closely to the prefrontal cortex and is involved in cognitive functions. There are many reciprocal connections with the hypothalamus which allows it to play a part in the regulation of autonomic activity (Luu & Posner 2003). Lesions most consistently result in affective changes: patients are apathetic and unconcerned when significant events occur, such as making mistakes (Luu & Posner 2003).
The hippocampus lies medial to the lateral ventricle and is distinguishable by the pattern in which it is folded upon itself. It is involved in the consolidation of memory. The amygdala is situated in the medial part of the pole of the temporal lobe, just below the cortex. It consists of three groups of nuclei: the basolateral, corticomedial and central nucleus. Input to the amygdala comes from the neocortex of all the lobes of the brain, the hippocampus and the cingulate cortex. Information from the different sensory systems is received by the amygdala and integrated across sensory modalities (Fatterpekar et al 2002).
Frontal lobe
The frontal lobe is involved in personality and behavioural traits. It also contains the motor cortex which is involved in carrying out movement. The prefrontal cortex is involved in sensorimotor incongruence sensing to ensure motor outputs are congruent with intentions (Fink et al 1999). The right ventral area of the lateral prefrontal cortex monitors proprioceptive and visual feedback and is activated by discrepancies between these different sensory signals. A dorsolateral prefrontal area is activated when actions need to be actively maintained in the presence of such conflict between intention and sensory outcome (Fink et al 1999).