Objectives/indications
Children frequently present to the pediatric emergency department with symptoms of abdominal pain, vomiting, and diarrhea. While these symptoms are most often related to self-limiting illnesses, they may be presenting signs for more serious conditions. A thorough history and meticulous examination may still be insufficient to differentiate the common malady from the more serious diseases. Moreover, it is often difficult to obtain an accurate history and reliably localize pain in young children, and nearly impossible in non-verbal children. The treating physician is then left to decide on a route of possible laboratory investigations and imaging modalities. Ideally, this route would include non-invasive, accurate, and readily available tests.
Abdominal ultrasound is one such modality, which is valuable, rapid, and it has an excellent accuracy in children presenting with abdominal complaints. While there are many advantages, abdominal ultrasonography can be a challenging exercise for various reasons. Intestinal peristalsis and intraluminal bowel gas may obscure target structures, or create artifacts that mimic other findings. Imaging a child presents other specific challenges related to the patient’s age and emotional maturity. Intants have a rapid physiologic respiratory rate in infants, which, when compounded by irritability and crying, makes abdominal structures move rapidly in and out of the sonographer’s viewing field. Moreover, a child in pain, or who is anxious, may not cooperate with the examination. It is of utmost importance to make the examination as comfortable as possible for the patient. This may be accomplished by using sucrose water solutions and warmed gel for infants, utilizing child-life specialists to explain the examination to younger children, having a caregiver at the bedside, and always paying particular attention to pain management.
While the majority of studies on utilizing ultrasonography for abdominal pathology have been performed in the radiology suite, there is a rapidly growing body of evidence that shows that point-of-care ultrasound can be rapidly used at the bedside as an effective screening and diagnostic tool. Point-of-care ultrasonography may be utilized to identify important abdominal pathology that causes bowel obstruction. The most common causes of bowel obstruction in children that are discussed in this chapter are: intussusception, acute appendicitis, and pyloric stenosis.
Intussusception
Background
Intussusception is the most common cause of intestinal obstruction in young children worldwide. Intussusception describes the process whereby one segment of bowel telescopes into the immediate distal segment. The proximal segment of bowel, the intussusceptum, telescopes into a distal segment, the intussuscipiens. The attached mesentery gets pulled along with the loop of bowel, resulting in constriction of venous outflow and impaired arterial perfusion. If left untreated, intussusception leads to bowel wall necrosis, perforation, and, rarely, death. This may occur throughout the lower gastrointestinal tract; however, the most common location is ileocolic. It occurs most often between three months and six years of age, with a peak presentation late in the first year of life. Incidence is 1–4 / 1000 live births, with males having a fourfold risk. The etiology of most cases of intussusception is unknown. Intussusception presenting outside the typical age range carries suspicion for an anatomic lead point. Up to 10% of cases have an identifiable lead point, such as a Meckel’s diverticulum, an inverted appendiceal stump, an intestinal polyp, lymphoma, or intestinal duplication. Patients with cystic fibrosis and Henoch–Schönlein purpura are predisposed to developing intussusception.
The typical clinical presentation is a previously healthy child who has developed sudden severe bouts of colicky pain or irritability interspersed with periods of lethargy. These symptoms are classically accompanied by vomiting, and guaiac-positive or grossly bloody stools. The classic finding of “currant jelly” stool represents a late finding (Figure 10.1). The triad of colicky abdominal pain, bloody stool, and vomiting is seen in less than 40% of patients, and 20% will present without pain. On physical examination, more than half of patients will have a palpable “sausage-shaped” mass in the right upper quadrant. Making the correct diagnosis is dependent on a high index of clinical suspicion. The initial imaging study is often abdominal radiography which may reveal a small-bowel obstruction (Figure 10.2a), a right upper quadrant soft tissue mass, a “target” or “crescent” sign (Figure 10.2b), where the terminal end of the intussusceptus meets with colonic air. Radiography can be normal in up to 45% of patients with proven intussusception, diagnostic in only 20–30%, and clinically unhelpful in 50% of cases. The most important finding on a plain film is the presence of free air, which indicates bowel wall necrosis and perforation and is an absolute contraindication to non-surgical reduction. Ultimately, the study of choice is an air or contrast enema, which is both diagnostic and therapeutic. In order to reduce the telescoped segment and restore perfusion, an air or contrast enema is performed. High pressure is administered through a tube in the rectum, which creates a pressure gradient until free passage of contrast or air is seen in the ileum. In cases where contrast or air enema is unsuccessful, surgical reduction is required and involves manual reduction or bowel resection. It is important to note that there is a 10% recurrence rate with enema-reduced intussusception, vs. 3–5% with surgical reduction. For this reason, children are generally admitted to the hospital for observation following reduction. However, it is important to note, almost half of the recurrences occur greater than 48 hours later.
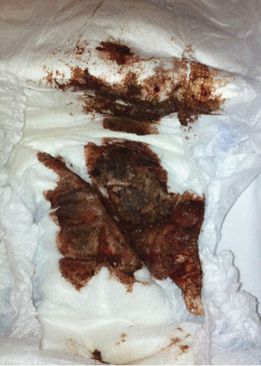
Figure 10.1 Currant-jelly stool, which is a classic finding in intussusception, but is rarely seen.
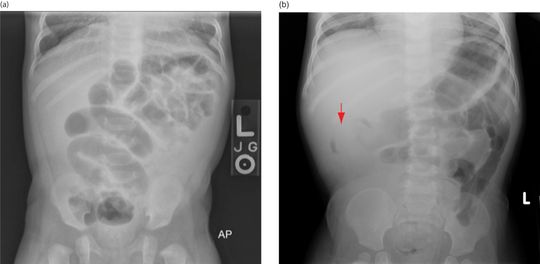
Figure 10.2 Radiographs in intussusception. Often, plain radiographs are nonspecific or normal. (a) Signs of obstruction may also be seen. (b) A classic finding is the “crescent” sign (arrow) at the location of the intussusception.
Ultrasonography has become increasingly utilized as a useful screening modality in the workup of intussusception. Several studies have been performed investigating formal ultrasonography, with a sensitivity of 98–100% and specificity of 88–100%. A recent study of 640 patients in Vietnam showed a sensitivity of 97.5% and specificity of 99%. It is suggested that utilizing ultrasound as a first-line diagnostic modality would decrease radiation-induced malignancy by 79.3 and 59.7 cases per 100,000 male and female children, respectively. Since the sensitivity and specificity of ultrasonography are both approaching 100%, it serves as an ideal screening tool, amenable to use in the emergency department. Riera et al. recently prospectively studied point-of-care ultrasound performed by pediatric emergency physicians with limited training. Point-of-care ultrasound had a sensitivity of 85%, specificity of 97%, NPV of 97%, and PPV of 85%.
Anatomy
When imaging the gastrointestinal (GI) tract, it is important to be familiar with the four histologic layers: (1) the luminal mucosa, (2) submucosa (including the muscularis mucosa), (3) muscularis propria, and (4) serosa (Figure 10.3). Intussusception in the typical pediatric age range involves the invagination of the ileum into the cecum, termed an ileocolic intussusception (Figure 10.4). Sometimes this process starts as ileoileal and then ileocolic, when there is first a small-bowel intussusception which then invaginates.
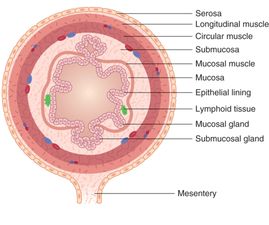
Figure 10.3 Bowel wall layers. (1) The luminal mucosa (small area of columnar cells noted secondary to mucosal destruction), (2) submucosa (including the muscularis mucosa), (3) muscularis propria, and (4) serosa. Artwork created by Emily Evans © Cambridge University Press.
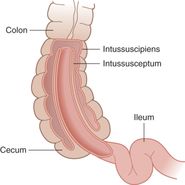
Figure 10.4 Intussusception. Intussusception involves the invagination of bowel layers: the intussusceptum into the intusscipiens. Artwork created by Emily Evans © Cambridge University Press.
Technique
Point-of-care questions
Transducer selection and orientation
Using a linear, high-frequency (5–10 MHz) transducer, the examiner should start in a transverse view at the level of the hepatic flexure (Figure 10.5a). From this position, identify the transverse and ascending colon, then trace down the ascending colon to the cecum and the level of the ileocecal valve.
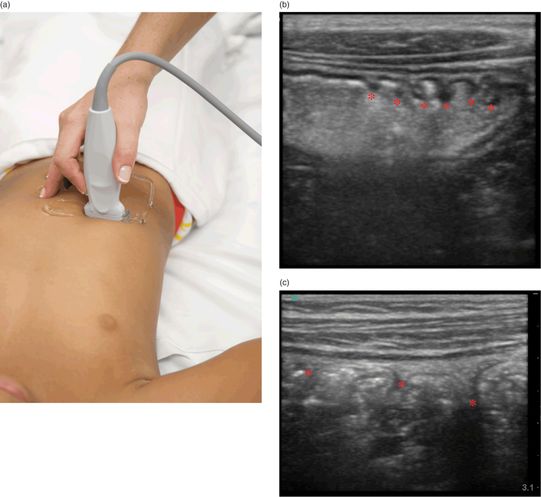
Figure 10.5 The evaluation for intussusception. (a) Transducer placement, transverse view with the indicator oriented towards the patient’s right side. Scanning is performed from the transverse to ascending colon, down to the cecum. (b) Ultrasound image, transverse view of the stomach showing hyperechoic gastric contents and the undulating gastric rugae (*). (c) Sagittal view of the ascending colon visualizing colonic haustra (*).
Patient position and preparation
The ultrasound examination begins with the patient in a supine position. Using pre-warmed gel and distraction techniques will help alleviate the anxiety and discomfort in the young patient. The scan can be attempted with the child in a parent’s lap if need be.
Ultrasound imaging
The ascending colon can be identified as the most lateral bowel structure on the right side of the abdomen. Visualization of a normal colon may be obscured by gas or fecal material. In the fluid-filled colon, haustral markings may be noted. Sonographically, normal bowel has alternating hyperechoic and hypoechoic structures. In cross-section, this creates a “target” or “bull’s eye” appearance with, potentially, five distinct layers representing the mucosa, muscularis mucosa, submucosa, outer circular muscularis, and serosa. The number of visible layers depends on the degrees of inflammation or distention present. Normal bowel wall thickness ranges from 3 to 5 mm in diameter. Sonographically, other anatomic features of normal bowel include gastric rugae, small-bowel valvulae conniventes, or colonic haustra (Figure 10.5b,c).
The visualization of an empty ileocecal valve can help to rule out an ileocecal intussusception. The identification of an intussusception consists of visualizing an oval, target-shaped mass typically seen at the hepatic flexure that was initially described in 1977 by Burke. The “target” or “doughnut” appearance is accentuated by the redundant alternating appearance of hyperechoic–hypoechoic bowel wall, stacked within one another with trapped mesentery inside (Figure 10.6a,b). Moving the transducer into a long-axis view produces the “hayfork” (Figure 10.6c,d) or “pseudokidney” sign (Figure 10.6e), which describes the invagination of the intussusceptum into the intussuscipiens. Mesenteric lymph nodes (Figure 10.7a) and fluid within or around the intussusception may also be seen (Figure 10.7b). There may also be secondary signs of small-bowel obstruction (Figure 10.7c,d). In addition, there are several signs seen on ultrasound that may predict failure of non-operative management. These include: large amounts of trapped fluid within the intussusception, poor or no blood flow on color Doppler imaging, multiple lymph nodes greater than 1 cm in diameter within the intussusceptum, or an outer wall thickness of greater than 1 cm (Figure 10.7e).
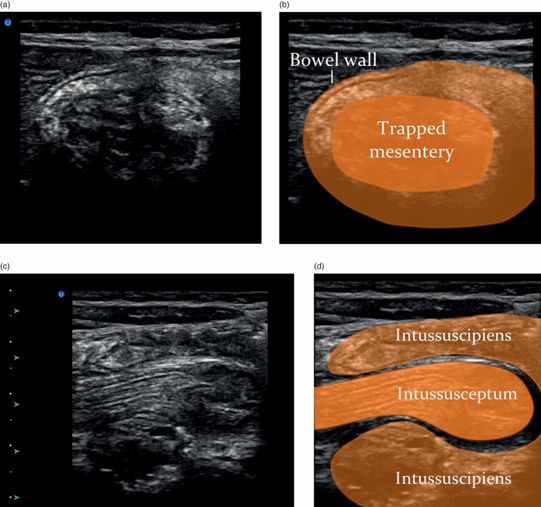

Figure 10.6 Intussusception. (a) Transverse view of an intussuception has layers of bowel around trapped hyperechoic mesentery in the center. (b) The transverse view is referred to as the “target” sign. (c) The longitudinal view of an intussusception shows the invagination of the intussusceptum into the intussuscipiens. (d) The longitudinal view is referred to as the “hayfork” sign. (e) The longitudinal view is also referred to as the “pseudokidney” sign. Illustrations by Laura Berg, MD.
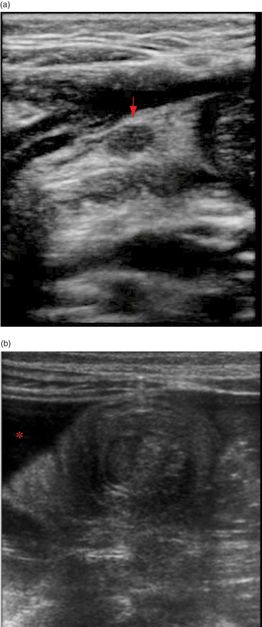
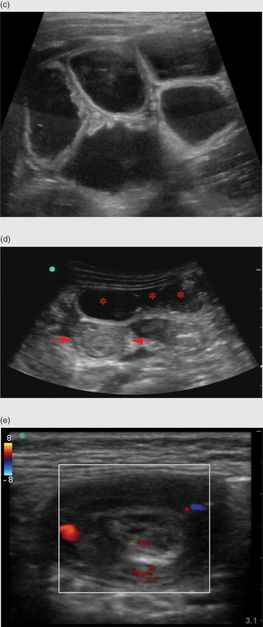
Figure 10.7 Additional findings in intussusception. (a) Mesenteric lymph nodes (arrow). (b) Fluid (*) surrounding the intussusception. (c) Dilated loops of small bowel associated with a small-bowel obstruction. (d) Dilated loops of bowel (*) anterior to an ileocolic intussusception (arrows). (e) Normal color Doppler of the bowel wall in an intussuception. The absence of flow may indicate a reduced chance of non-operative reduction.
Ultrasound imaging: measurements
Measurements of the bowel wall or overall diameter are an important part of the abdominal examination. Transducer positioning should be in the ideal plane to avoid incorrect measurements giving falsely positive or negative findings depending on the planes of view. When the difference between positive and negative findings is measured in millimeters, correct positing is imperative. With intussusception, though there are size measurements that can be made (2.5–5 cm), the diagnosis is based on sonographic appearance alone.
Scanning tips
Pitfalls
- Ultrasound examinations are largely operator dependent. Despite this, studies have shown that junior radiology residents have a similar accuracy when compared with their radiology attendings in identifying intussusception by ultrasound.
- False-positive studies may arise from other etiologies of bowel wall thickening that may give a “pseudokidney” sign, including enterocolitis, inflammatory bowel disease, and an intramural hematoma.
- Stool may be mistaken for an intussusception. However, stool does not exhibit circumferential layering of the bowel wall, as is seen with intussusception.
- False-positive or false-negative studies may be found in a child with intermittent self-reducing intussusception.
- When the depth is not properly adjusted, the intussuception may be missed. It is important to increase the depth enough to visualize the anterior and posterior walls of the intussuception.
- There may be misidentification of the position of the intussusception; finding it within the small bowel instead of the typical ileocolic position.
- Ileoileal intussusceptions may be missed by ultrasonography.
Tips to improve scanning
Appendicitis
Background
Appendicitis is the most common abdominal emergency requiring surgery in children, with an incidence of about 80,000 cases per year in the United States. Its peak presentation is in the second decade of life, with males being affected more often than females. Acute appendicitis results from obstruction of the appendiceal lumen, promoting distal inflammation and distention (Figure 10.8). This process compromises perfusion, facilitating microbial invasion into the appendiceal wall, ultimately leading to perforation, peritonitis, and abscess formation. Rates of ruptured appendicitis range from 10–20% in adolescents, to as high as 100% in patients less than four years of age. This difference is attributed to both earlier and more typical presentations in the adolescent age group, compared to the younger, non-verbal children.
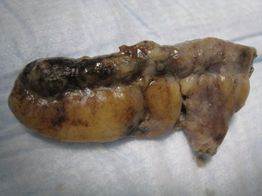
Figure 10.8 Appendicitis. Pathology specimen in an inflamed appendix with a necrotic tip, consistent with acute appendicitis.
The classic presentation of appendicitis begins with vague periumbillical abdominal pain that worsens over time and localizes to the right lower quadrant. There is associated vomiting, anorexia, and fever. The symptoms typically evolve over a period of 24–48 hours, with delays in diagnosis greater than 48 hours associated with an increased risk of perforation. Unfortunately, only about 50% of patients present with the classic features of appendicitis. Other signs or symptoms may also be present. Abdominal inflammation may cause irritation of nearby structures such as the bladder or sigmoid colon, causing dysuria or diarrhea, respectively. Ultimately, the localization of pain to the right lower quadrant arises secondary to local irritation of the overlaying peritoneum, while a retrocecal appendicitis may localize pain to the hip, mimicking a septic arthritis. While late findings of peritonitis are typically unmistakable, treating physicians can miss the diagnosis with earlier presentations. The diagnosis is further complicated in children who are poor historians, and in adolescent females who may have presentations that overlap with potential gynecologic pathology. Several clinical scoring systems have been developed, such as the Alvarado score and the Pediatric Appendicitis score, which take into consideration a patient’s clinical presentation and laboratory studies. While these scoring systems may help guide the clinician, they cannot be solely relied upon in the diagnosis or exclusion of appendicitis. Laboratory investigations are often used to aid in diagnosis, and may include but are not limited to a complete blood count, C-reactive protein (CRP), and urine analysis. While an elevation in the white blood cells or absolute neutrophil count suggests appendicitis, patients may not have significantly elevated levels. In addition, the irritation of the ureter or urinary bladder from an acutely inflamed appendix may cause white and red blood cells to appear in the urine, falsely suggesting a urinary tract infection. While unambiguous cases may proceed to the operative suite, equivocal cases often lead to further imaging studies.
In 1986, Puylaert described the technique of graded compression ultrasonography for the evaluation of acute appendicitis. The author based the diagnosis of appendicitis only on the presence or absence of a visible appendix. In a study of healthy patients without any abdominal complaints, Rettenbacher et al. showed that 23% of subjects had an appendiceal diameter greater than 6 mm, and 9% were greater than 7 mm. Therefore, a measurement greater than 6 mm does not automatically indicate acute appendicitis. In another study, the same group found that a consistently ovoid, as opposed to a completely or distally round, appendix correlated with a normal appendix. In the case of an ovoid-appearing appendix, the relevant measurement should be made at the narrowest part of the oval. The overall sensitivity of an ultrasound examination ranges between 50 and 100%, and specificity is between 88 and 99%. Therefore, a negative study cannot reliably rule out acute disease. Further complicating matters is that visualization of the appendix varies widely from 22 to 98%. Visualization may largely be operator dependent, but also dependent on patient characteristics. Recent studies show that children with a BMI of greater than 85, with a clinical probability of less than 50% of having appendicitis, had a 58.1% probability of having an inaccurate or equivocal ultrasound examination. Most recently, Mittal et al. performed a large pediatric cohort study of radiologist-performed ultrasound. Results showed an overall sensitivity and specificity of 72.5% and 97% respectively, which is consistent with prior studies. However, in separating out those cases where the appendix was clearly identified, the sensitivity increased to 97.9%, but with a slightly lower specificity of 91.7%. Despite these drawbacks, institutions looking to reduce radiation exposure have proposed using ultrasonography as the initial diagnostic modality, with CT only in negative or equivocal cases. This staged approach has resulted in acceptable rates of negative appendectomies and significant reductions in CT utilization. With a staged ultrasound protocol, the sensitivity and specificity were shown to be 98.6% and 90.6% respectively, with a negative appendectomy rate of 8.1% and a missed appendicitis rate of less than 0.5%. Further, upon review of recent published evidence, in 2010 the American College of Radiology reaffirmed that ultrasonography should be the first-line imaging study in children under 14 years of age and in pregnant women.
Recently, Fox et al. applied this technology to the bedside. Prospectively enrolled patients had a point-of-care ultrasound performed by attending physicians and residents in the emergency department. Sensitivity for the point-of-care ultrasound was 65% and specificity was 90%, with a PPV of 84% and an NPV of 76%. Since point-of-care ultrasound has a low sensitivity, it should only be utilized as a “rule in” modality, but not to rule out disease. Further larger-scale studies need to be performed as clinicians gain further experience in utilizing point-of-care ultrasound for appendicitis.
Anatomy
The vermiform appendix is a blind-ending tubular structure that arises from the cecum, 1–2 cm from the ileocecal valve (Figure 10.9a). In infancy, it has a more conical shape that becomes characteristically tubular by one to two years of life. The length can range from 2 to 20 cm, and the tip may lie in variable positions within the abdominal-pelvic cavity (Figure 10.9b). Blood to the appendix is supplied by the appendicular artery, a branch of the ileocolic artery.
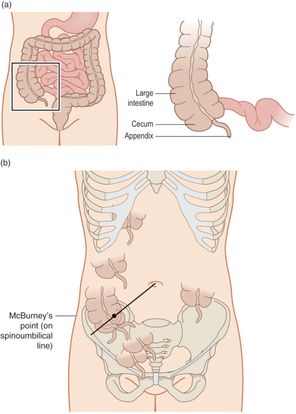
Figure 10.9 The appendix anatomy. (a) Illustration of the appendix anatomy. (b) The appendix has various orientations in the body, making it especially challenging to identify. Artwork created by Emily Evans © Cambridge University Press.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








