Fig. 25.1
Budding mucosa (arrow) below the VICRYL mesh in an open abdomen
In this clinical presentation, the fistula presented is termed an “entero-atmospheric fistula” (EAF). It is a fistula without an epithelialized tract and the opening is exposed to air (hence the term “atmosphere”) instead of skin. This type of fistula is very different from the traditionally discussed entero-cutaneous fistula (ECF), because EAF presents a much more clinical challenge in terms of patient’s critical illness, controlling fistula effluence, and the unlikely spontaneous closure of the fistula. However, there have been several prior case reports of spontaneous fistula closure of the EAF using various techniques [2–4]. These will be further discussed later.
Etiology
Etiology for EAF is somewhat different from ECF, which often discussed in terms of intrinsic versus extrinsic factors. Intrinsic factors included conditions such as an inflammatory bowel disease and diverticulitis. Extrinsic factors included postoperative anastomotic leak and iatrogenic bowel injury. On the contrary, EAF arises mostly from extrinsic factors, usually in association with open abdomen. For example, the fistula can arise from an unrecognized iatrogenic bowel injury that is delay detected during a postoperative course (Fig. 25.2), an anastomotic leak (Fig. 25.3), a significant trauma that is associated with significant loss of abdominal wall domain (Fig. 25.4), or a complication of open abdomen from desiccation (our case scenario). Therefore, whether the open abdomen is intentional or unintentional, the open abdomen is definitely a risk factor for EAF development.
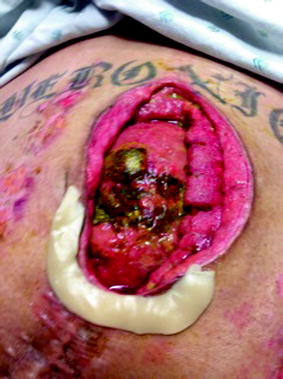
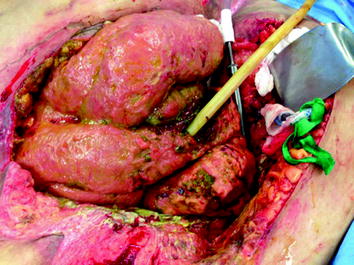
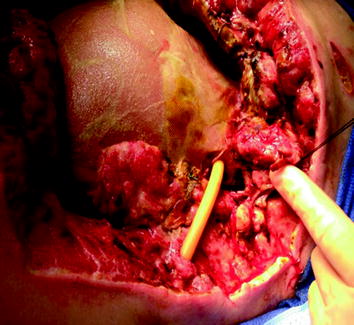

Fig. 25.2
Atmospheric small-bowel fistula arises from unrecognized iatrogenic small-bowel injury in a 46-year-old male who underwent ventral hernia repair and lysis of adhesion

Fig. 25.3
Atmospheric small-bowel fistula arises from an anastomotic leak 10 days postoperatively in a morbidly obese patient with a body mass index of 75

Fig. 25.4
Atmospheric (gastric and small bowel) fistula arises from open abdomen due to gunshot wound that is associated with a significant loss of abdominal domain
Classification
In ECF, a general classification of fistula is often based on effluent output per 24 h. A high output is 500 cc per 24 h, while a low output is less than 200 cc, and a moderate output is between 200 and 500 cc. The classification can also be based on the anatomical location such as gastric versus small bowel versus large bowel. These classifications may have some predictive roles of possible spontaneous fistula closure. These two schematic classifications can certainly be applied to EAF, but the relationship of fistula classification and spontaneous fistula closure outcome has never been studied because EAF is more rare. All reports on EAF [2–10] have all been either a case report or case series with a very limited number of patients; therefore, any definitive conclusion is very difficult. Furthermore as mentioned previously, because of the lack of fistula tract in EAF, the spontaneous fistula closure is most unlikely.
Management
The general principle of ECF management can still be applied to EAF. These include sepsis source control, fluid and electrolytes monitoring with appropriate replacement, nutritional support, and wound/skin care and protection. However, a general notion in ECF that the fistula might close spontaneously after ruling out distal obstruction and foreign body may not be equally applicable to EAF, because EAF has different etiologies, lacks the fistula track, and is often associated with a complex and austere intra-abdominal environment that often arises from the intra-abdominal catastrophe. EAF is also often associated with significant abdominal wall domain loss and/or defect that requires a major abdominal wall reconstruction during the reoperation.
Although the hope in managing EAF is that one would like to achieve a spontaneous fistula closure, but in reality knowing that spontaneous fistula closure is unlikely, the true goal in EAF management is to control the fistula effluence well enough that a tissue coverage; i.e., skin graft can be accomplished. Then a plan to return at a later date to deal with the fistula take-down and reconstruction of the abdominal wall defect can be implemented. Various surgical techniques [2–10] in dealing with EAF have been reported in the literature. Most cases were anecdotal author’s personal case reports. Some were case series. The summary of these reports including technique is listed in the table (see Table 25.1). The main idea among these various reports can be grouped as either attempts to close the fistula primarily [2–4], or to control the effluence with tissue coverage using skin graft. Our own authors at some points have attempted all of these techniques including a recently reported “Fistula Patch” by Wang et al. [10]. The authors experienced different results (personal experience) from what has been reported. As a result, authors have also come up with our own approach, and we used a modified Hirschberg’ Malecot tube technique [5] that we will further discussed later.
Table 25.1
Various techniques used to close the fistula or control the effluence of the fistula
Authors | Technique | N | Comment |
|---|---|---|---|
1. Jamshidi and Schecter [2] | Biological dressing | N = 7 | Success closure 5/7 patients |
2. Girard et al. [3] | Biological dressing + fibrin glue | N = 1 | Success closure |
3. Sarfeh and Jakowatz [4] | Primary repair + STSG | N = 1 | Success closure |
4. Al-Khoury et al. [5] | Malecot tube + VAC | N = 1 | |
5. Subramaniam et al. [6] | Floating stoma | N = 7 | |
6. Cheesborough et al. [7] | Red rubber tube + STSG | N = 7 | |
7. Gunn et al. [8] | Fistula VAC | N = 15 | Success closure 11/15 patients |
8. Layton et al. [9] | Baby nipple + VAC | N = 1 | |
9. Wang et al. [10] | Fistula patch | N = 11 | |
10. Kulvatunyou et al. (current series) | Malecot tube + advanced flap ± VAC | N = 3
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





