FIGURE 1 Potential sites of sensitization mechanisms associated with visceral pain. On left is a schematic diagram of the normal physiological state of an organism. On the right are indicated potential sites for sensitization mechanisms which are listed to the far right. Table 1 indicates more precise mechanisms located at these sites.
The overriding hypothesis that has driven studies in our laboratories for the last three decades is that the conscious perception of visceral pain is associated with the activation of at least two separate subsets of visceroceptive, nociceptive, second-order neurons. These neurons can broadly be categorized as (1) second-order neurons which are associated with focal activation within the spinal cord and (2) second order neurons which are associated with widespread activation within the spinal cord – MNN neurons. Widespread activation is relatively easy to mechanistically accomplish and can consist of either short or long axonal excitatory connections that are associated with a degree of reciprocity. Neuronal lattices with these features have been commonly identified to exist within craniospinal structures and have been described as propriospinal excitatory connections and intersegmental connections. Early neurophysiological studies of spinal visceroceptive neuronal interconnectivity immediately identified such interconnections [12,13]. In contrast, focal activation of spinal neuronal structures requires either a precise set of excitatory connections from the viscera and/or the presence of feedback inhibitory systems that focus the excitatory effects of transmission signals to a specific spinal location. Painful stimuli can activate such feedback inhibitory systems, a general phenomenon which we have previously referred to as nocigenic inhibition (NI) [26,27]; with specific cases described as conditioned pain modulation [43], diffuse noxious inhibitory controls [8–10,43], propriospinal inhibitory circuits and segmental inhibitory phenomena [16,17]. Based on our interpretation of neuroanatomic and functional studies and building on concepts championed by Lebars and colleagues [8–10], we have chosen to focus on NI influences as predictors of sensory function and so have utilized neuronal susceptibility to pain-activated inhibitory control systems as a defining functional characteristic. Our overriding hypothesis has therefore simplified to the assertion that visceral pain is due to the activity of two spinal dorsal horn neuronal subgroups: Inhibition Susceptible neurons (IS, Fig. 2) and MNN neurons (Fig. 3).
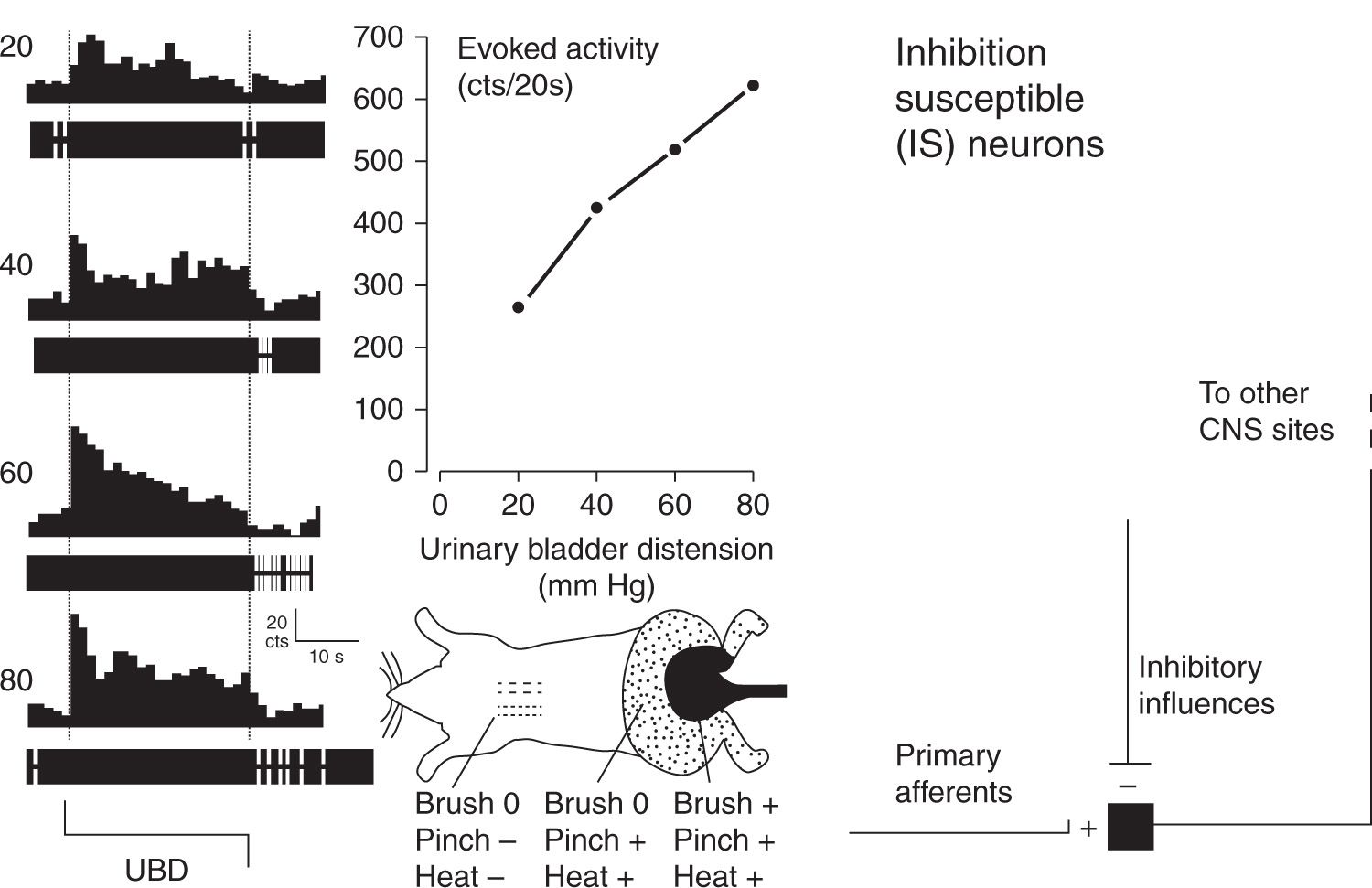
FIGURE 2 Typical example and schematic description of an Inhibition Susceptible (IS) neuron. At far left are peristimulus-time histograms and oscillographic tracings associated with an L6/S1 spinal dorsal horn neuron excited by graded intensities of urinary bladder distension (UBD; pressure of distension in mm Hg indicated at left). Dotted lines indicate onset and cessation of distending stimulus. The center upper graph indicates graphically the excitatory response of the same neuron – number of action potentials were counted (cts) for the 20 period of UBD and spontaneous activity subtracted out to give a measure of evoked activity. The center bottom graph is a cartoon indicating convergent cutaneous receptive field of neuron – notably pinch of the midscapular skin at a noxious intensity produced inhibition (indicated by “-”) whereas light touch brush and noxious pinch and heat produced excitation (indicated by “+’; no response “0”) of the neuron when presented in lumbosacral fields. At far right is schematic diagram indicating essential features of IS second order neurons. (Adapted from Ness TJ, Castroman P. Evidence for two populations of rat spinal nociceptive neurons excited by urinary bladder distension. Brain Res 2001; 923:147–156).
Quantitative characterization studies that have assessed for NI effects in visceroceptive neurons suggest that an important neurofunctional difference between visceral pain and cutaneous pain is that somatic-only (not viscerosomatic) nociceptive neurons are almost exclusively IS neurons [8–10,32] whereas a majority of viscerosomatic nociceptive neurons are non-IS neurons with a ratio slightly greater than 50:50 compared to IS neurons in quantitative studies of dorsal horn neurons excited by either colorectal distension or bladder distension [20,26]. Evidence that non-IS neurons are MNN neurons is that they are typically excited by noxious stimuli applied to distant (heterosegmental) parts of the body often including the entire body surface. It is the differential susceptibility of IS and MNN neurons to other inhibitory and facilitatory influences that may explain the necessity of central sensitization processes for the experience of visceral pain.
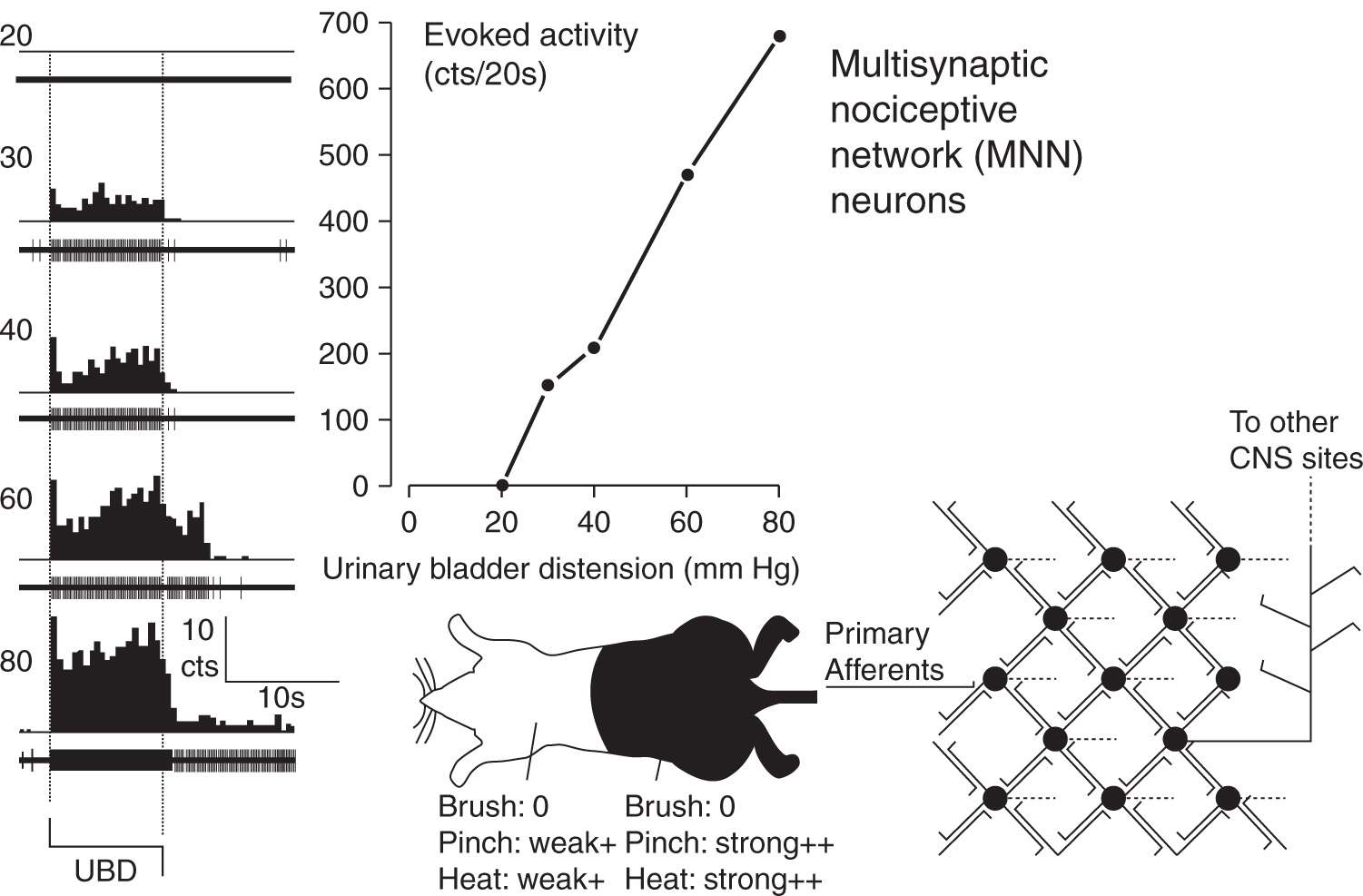
FIGURE 3 Typical example and schematic description of Multisynaptic Nociceptive Network (MNN) neuron. At far left are peristimulus-time histograms and oscillographic tracings associated with an L6/S1 spinal dorsal horn neuron excited by graded intensities of urinary bladder distension (UBD; pressure of distension in mm Hg indicated at left). Dotted lines indicate onset and cessation of distending stimulus. In center upper graph indicates graphically the excitatory response of the same neuron – number of action potentials were counted (cts) for the 20 period of UBD and spontaneous activity subtracted out to give a measure of evoked activity. In center bottom is a cartoon indicating convergent cutaneous receptive field of neuron – this neuron had strong excitatory responses to noxious pinch and heat (indicated by “++’) when these stimuli were presented in lumbosacral fields; presentation in distant heterosegmental sites produced weak excitatory responses. In this neuron, non-noxious brush produced no response (indicated as “0”) of the neuron. At far right is schematic diagram indicating essential features of MNN second order neurons which have reciprocal excitatory connections with multiple other spinal neurons. (Adapted from Ness TJ, Castroman P. Evidence for two populations of rat spinal nociceptive neurons excited by urinary bladder distension. Brain Res 2001; 923:147–156).
DESCRIBING THE SUBJECT
Studies of Visceroceptive Neurons Using Nocigenic Inhibition-Related Criteria
In our initial studies of visceral nociceptive neurons, which used the visceral stimulus of colorectal distension, there were neuronal subgroups that could be differentiated based on “temporal” characteristics of their responses. One subgroup abruptly terminated their excitatory discharges following cessation of the distending stimulus and hence were called ABRUPT neurons [22,23]. This was in contrast to a separate subgroup of neurons excited by colorectal distension that had a sustained after discharge following cessation of the distending stimulus and hence were called SUSTAINED neurons [22,23]. Only after introduction of neuronal characterization methods that included NI assessments did it become clear that all ABRUPT neurons were IS neurons [26]. The simplest explanation for their “abrupt” temporal characteristics was that a negative-feedback NI system was being activated by the visceral stimulus. The end result of this feedback inhibition would be an adapting response with a sharp reduction in neuronal activity as soon as the primary afferent excitatory signaling ceased. The same inhibitory phenomenon was not generally observed in SUSTAINED neurons, most of which were non-IS neurons. In many cases these lumbosacral neurons were excited by neurologically distant noxious stimuli (nose or front paw pinch in rats)[26]. This activation by heterosegmental stimuli gives strong evidence that these neurons received multisynaptic input making them at least third- or fourth-order spinal neurons, in addition to being second-order neurons. By our definition that made them MNN neurons. Identification of these neurons in spinally-transected rats indicated that the “network connections” did not require a brainstem circuit. When later utilizing urinary bladder distension as a noxious visceral stimulus, use of susceptibility to NI to determine neuronal subclasses became our routine as it resulted in empirically precise differentiation of neurons, in that individual neurons either met criteria or did not meet criteria in an unambiguous fashion [20].
IS neurons have been demonstrated to have many different characteristics than MNN neurons apart from their susceptibility to NI. In comparison with MNN neurons, IS neurons require lower pressures of visceral distension to be activated. They also have smaller convergent cutaneous receptive fields with distinct “inhibitory-surround” characteristics. That is, noxious stimuli applied outside the areas of excitation produce inhibition [20,22,23,26]. IS neurons are less inhibited than MNN neurons by systemic administration of traditional analgesics, such as opioids and lidocaine [18–20,24], but more inhibited by NMDA receptor antagonists [1]. Further, inflammation of visceral structures (e.g. colon, bladder) results in quantitatively less vigorous excitatory responses in samples of IS neurons [21,28,29] (Fig. 4 upper) as does the presentation of an experimental stressor [39] (Fig. 5 – upper). Stated in a converse fashion, this means that the MNN neurons, which represent a majority of visceroceptive neurons, when compared with IS neurons have larger convergent cutaneous receptive fields (often total body), are more inhibited by traditional analgesics and demonstrate more robust responses following manipulations known to increase clinical pain such as inflammation and stress/anxiety. Notably, differential modulation of the IS and MNN neurons occurred whether the “trigger” for sensitization was either a clearly primary afferent-related mechanism (inflammation) or due to supraspinal mechanisms (stress/anxiety). These modulators also increase visceral nociceptive reflexes in a similar fashion [36,38,40]. What is particularly notable when contrasting these two modulations is that IS neurons demonstrated susceptibility to stress-induced inhibitory influences in addition to the NI manipulations used to define that neuronal group. In contrast, MNN neurons demonstrated robust responsiveness to stress-induced facilitatory mechanisms. It is likely that inflammation also resulted in central facilitatory mechanisms that impacted on MNN function, but the effect of central facilitation is difficult to dissociate from the increased primary afferent inputs produced by inflammatory processes.
Features of Visceral Pain
So how might a difference in the populations of neurons excited by nociceptive stimuli help us understand other features of visceral pain? This type of pain has as its hallmark feature a diffuse localization. Much of the diffuse localization can be explained by the diffuse point of entry of primary afferents into the CNS from any particular visceral site. Cell bodies in the dorsal root ganglia spanning from midthoracic to lower sacral sites are labeled when pelvic organs are injected with neuronal tracers (e.g. [23]). Further, Sugiura et al [42] demonstrated that once a single visceral afferent C-fiber enters the spinal cord it often branches and may form weak synaptic contact with more than 10 spinal segments with branches extending into multiple ipsilateral and contralateral spinal laminae. In contrast, single C-fiber afferents from cutaneous structures often form tight baskets of input to a limited number of second-order neurons within limited ipsilateral laminae of single spinal segments [42
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






