The History of Regional Anesthesia
David L. Brown
B. Raymond Fink
The popularization of regional anesthesia was not possible until two events occurred. First, a local anesthetic was required, and second, advanced understanding of infectious agents was needed. In 1886, the introduction of cocaine by Koller provided the answer to the first required event, and the increased understanding of infectious complications was made possible by the introduction of the theories of Lister in the 1870s (1). The sequence of these requirements was essential in allowing regional anesthesia to progress since, had physicians progressed to spinal or other regional anesthetics prior to introduction of asepsis to the practice of medicine, it seems likely that regional anesthesia would have been significantly delayed or even prevented from progressing into surgical practices. To most completely understand whence our regional practices have developed, it is helpful to examine a number of issues, including the physiology of neural transmission; the development of local anesthetics (especially cocaine); the use of infiltration anesthesia, intravenous (IV) regional anesthesia, spinal anesthesia, obstetric, and epidural anesthesia; paravertebral anesthesia; and the maturation of the specialty of regional anesthesia within the larger specialty of anesthesiology.
Physiology of Neural Transmission
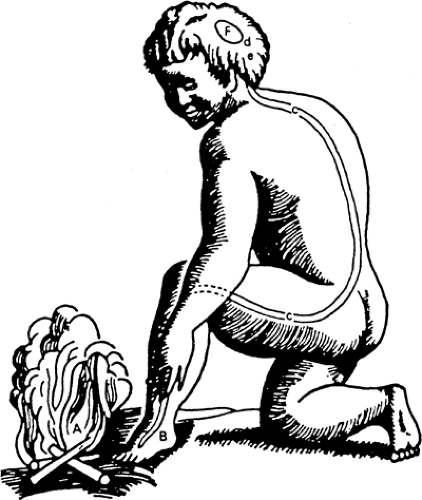
Fundamental to modern neural blockade and regional anesthesia is the concept that sensory block is accomplished by pharmacologically interrupting specific nerve fibers, amenable, in principle, to modulation or interruption along the nerve’s pathway. This outlook may be traced back to developments in the study of physiology that finally supplanted the view first expressed by Plato and Aristotle that pain, like pleasure, is a passion of the soul—that is, an emotion and not one of the senses (Table 1-1). Philosophical changes from the great scientific revolutions of the 18th century and the birth of biology gradually, although not entirely, effaced the religious connotations of pain in Western civilization (2). These philosophical revolutions of the 18th century were in part based on the mechanistic concepts of biologic function that Descartes developed during the 17th century. Descartes matured the concept of a neural connection from the periphery to the brain (Fig. 1-1). James Moore (1762–1860), a London surgeon (Fig. 1-2), used these mechanistic concepts to promote neural compression as a useful technique for the provision of surgical anesthesia (Table 1-2). As illustrated in Figure 1-3, Moore developed techniques for both upper and lower extremity nerve compression and wrote his monograph, “A Method of Preventing or Diminishing Pain in Several Operations of Surgery,” only after experimenting upon himself (2a,2b).
The doctrine of specific energies of the senses was first promulgated by Johannes P. Müller (1801–1858) in 1826 (3). This doctrine, although it did not posit specificity for the conduction of pain, initiated the movement of scientific thought toward analysis and classification of the specific characters of different nerves. Earlier, in 1803, Charles Bell defined important functions of the dorsal roots of the spinal nerves as distinct from those of the ventral roots, and he initiated a rigorous search for a more complete understanding of the sensory phenomena. Bell highlighted that dorsal root function is sensory and ventral root function is motor (4). In 1851, von Helmholtz succeeded in measuring the velocity with which the nerve impulse travels and opened the way for the development of modern electrophysiology (5).
The theory that pain was a separate and distinct sense was first definitely formulated by Moritz S. Schiff (1823–1861) (7). Rynd’s idea may be said to have foreshadowed both nerve block and, more remotely, opioid regional analgesia.
According to some accounts of the 1850s, Pravaz in Lyon and Wood in Edinburgh invented the syringe and hypodermic hollow needle, respectively (Table 1-2). A thorough sifting of the historical evidence (8) and independent reexamination of the sources support the following outline of the facts. In 1845, Rynd described the idea of introducing a solution of morphine hypodermically in the neighborhood of a peripheral nerve (7), with the intention of allaying neuralgic pain in that nerve. However, he introduced the solution not by syringe but by means of gravity, allowing it to enter passively through a cannula after removal of the trocar. The invention of the syringe is lost in the mists of several centuries preceding Alexander Wood (1817–1853) in 1853, following experiments in animals (10). Pravaz himself had used a syringe and trocar (trois-carre). Wood thus originated the practice of percutaneous subcutaneous injection to medicate locally a peripheral
nerve. His technique was adopted by C. Hunter and renamed hypodermic injection, ostensibly because Hunter had in view a different purpose, namely, systemic absorption of the drug.
nerve. His technique was adopted by C. Hunter and renamed hypodermic injection, ostensibly because Hunter had in view a different purpose, namely, systemic absorption of the drug.
Table 1-1 Chronology of Ideas Concerning Pain and Neural Blockadea | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||
Carl Koller (1857–1923) (12) conceived and attempted the direct application of an analgesic to the spinal cord, but a defective rationale and unserviceable technique stultified his approach to the management of chronic pain (Fig. 1-5). A deeper knowledge of the underlying
mechanisms was requisite. Understanding of these mechanisms remained relatively superficial until the era of electrophysiologic and neuropharmacologic microexploration following World War II.
mechanisms was requisite. Understanding of these mechanisms remained relatively superficial until the era of electrophysiologic and neuropharmacologic microexploration following World War II.
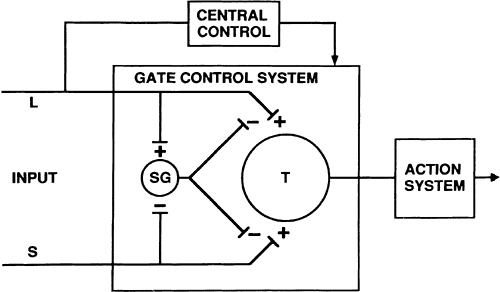
Melzack and Wall’s hypothesis that a spinal “gate” controls the cephalad transmission of nociception (13) was based on evidence suggesting that the intensity and quality of pain perceived do not bear a push-button, straight-through, one-to-one relationship to the intensity of the stimulus, but are instead determined by a multiplicity of physiologic and psychologic variables (Figs. 1-6 and 1-7). This led directly to the reintroduction of electrical stimulation as a method of treating chronic pain. Although their gate control theory has been shown to be conceptually incomplete in light of today’s understanding, it did provide the framework for most of the advances in understanding spinal cord nociceptive processing.
The search for the mechanism of opioid analgesia and opioid addiction resulted in Reynolds’ spectacular demonstration, in 1969, of the analgesic effect of electrical stimulation of the periaqueductal gray matter (14). This seminal discovery gave enormous impetus to pain research and led to the uncovering of a system of descending neurons that inhibit pain and are activated by opiate drugs acting at endorphinergic synapses. Brilliant experimental work by many researchers conceptualized analgesia via a direct spinal action of narcotics, a landmark advance from which important clinical developments have sprung (15,16,17). Further experimental studies have now been extended to define, using isolated nerve techniques, the concept of “neuroplasticity” and how multiple spinal cord receptors may be modulated by preemptive analgesia techniques (18). The progression of clinical applications sharply illustrates the process and value of basic medical research.
In the last century, World War II was a stimulus for regional anesthesia development; secondary to two factors: the many injured needing medical care and the introduction of lidocaine to regional techniques. The Seattle personalities Daniel C. Moore and John J. Bonica (Fig. 1-8) led the effort in the United States to grow both regional anesthesia and pain medicine care, with Moore primarily focused on regional anesthesia and Bonica on pain medicine. Both of these men were consummate physicians, and neither lacked confidence or compassion. Further, Moore’s interest was primarily clinical research on techniques. He was the primary encourager of the development of the American Society of Regional Anesthesia (ASRA), although not a founding member of the society. Bonica’s interest was to better understand matching regional techniques to patients experiencing chronic pain, and his interest ultimately led to the development and leadership of the International Association for the Study of Pain (IASP). Both of these men surrounded themselves with outstanding colleagues.
Cocaine
The mid 19th century was a period of growth in Western science and technology. In 1865, 6 years after the publication of
Charles Darwin’s epochal book, Lister opened a new era in surgery by applying Pasteur’s proof of nonspontaneous generation to the elimination of sepsis. Pflüger showed that the seat of respiration was in the tissues and not in the blood and, in 1882, the same year that produced the world’s first electrical power station (in New York), Ringer demonstrated the need for calcium and potassium salts to maintain the excitability of the heart. The establishment of the coal tar industry in Germany led to large-scale production of pharmaceuticals, of which the marketing of cocaine by Merck was one result. The year 1886 saw the introduction of steam sterilization of dressings by von Bergmann, and the year 1890, the use of surgical rubber gloves, initially for the purpose of protecting the hands of Halsted’s instrument nurse from disinfectant.
Charles Darwin’s epochal book, Lister opened a new era in surgery by applying Pasteur’s proof of nonspontaneous generation to the elimination of sepsis. Pflüger showed that the seat of respiration was in the tissues and not in the blood and, in 1882, the same year that produced the world’s first electrical power station (in New York), Ringer demonstrated the need for calcium and potassium salts to maintain the excitability of the heart. The establishment of the coal tar industry in Germany led to large-scale production of pharmaceuticals, of which the marketing of cocaine by Merck was one result. The year 1886 saw the introduction of steam sterilization of dressings by von Bergmann, and the year 1890, the use of surgical rubber gloves, initially for the purpose of protecting the hands of Halsted’s instrument nurse from disinfectant.
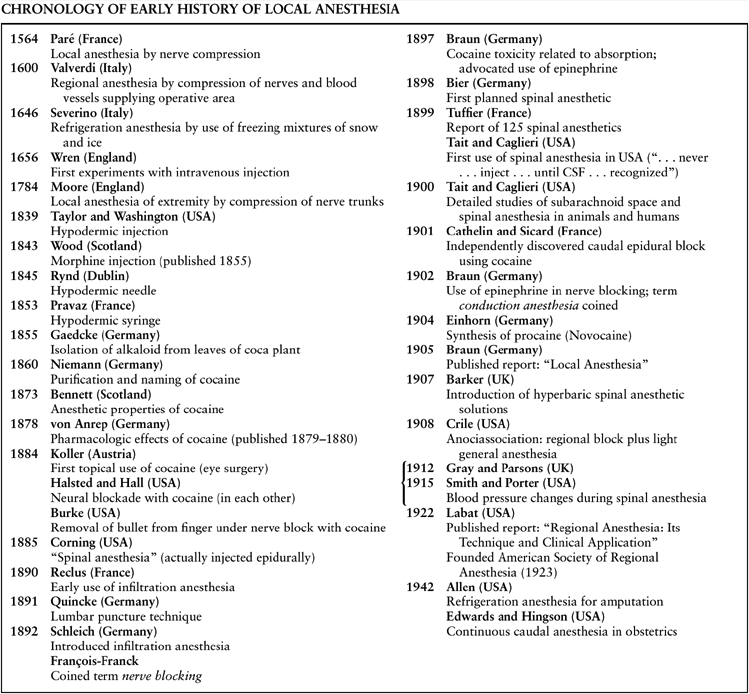
Table 1-2 Chronology of Early History of Local Anesthesia | |
|---|---|
|
Koller’s demonstration of ocular surface anesthesia with cocaine (11) had antecedents almost as numerous as those of general anesthesia 40 years earlier. Dr. Scherzer, an Austrian explorer and a member of an expedition to South America, returned with coca leaves to Vienna. Some were sent to Friedrich Wohler for analysis, and subsequently to his pupil, Albert Niemann. Niemann (1834–1861) was successful in isolating and naming the alkaloid from the leaves of Erythroxylon coca, as first recorded in 1860 in a report signed W. (for H. Wofler 1800–1882), which also related the passionate chewing of the leaves by the coqueros of Peru and the deleterious mental effects this had on them (19,20). Nobody paid a great deal of attention to the benumbing effects of cocaine on the tongue and the lips until the Peruvian army surgeon Moreno y Mayz remarked in 1868 that the sensory paralyzing effects of cocaine might be put to use in medicine (21).
A thorough pharmacologic investigation of the properties of the alkaloid in frogs was presented by von Anrep, a Baltic
surgeon. In 1880, von Anrep published an extensive article on the physiologic and pharmacologic effects of cocaine. It is clear that he understood that cocaine had a locally numbing effect on the tongue and that it dilated the pupil upon local application, and he did suggest that this drug might some day become of medical importance (22). He ended the report as follows: “The animal experiments have no practical application; nevertheless I would recommend trying cocaine as a local anesthetic in persons of melancholy disposition (22).” Plainly, von Anrep was most impressed by the stimulating properties of cocaine, and these seem also to have been uppermost in the mind of Sigmund Freud (1856–1939) when he suggested a study of the drug to Koller (23).
surgeon. In 1880, von Anrep published an extensive article on the physiologic and pharmacologic effects of cocaine. It is clear that he understood that cocaine had a locally numbing effect on the tongue and that it dilated the pupil upon local application, and he did suggest that this drug might some day become of medical importance (22). He ended the report as follows: “The animal experiments have no practical application; nevertheless I would recommend trying cocaine as a local anesthetic in persons of melancholy disposition (22).” Plainly, von Anrep was most impressed by the stimulating properties of cocaine, and these seem also to have been uppermost in the mind of Sigmund Freud (1856–1939) when he suggested a study of the drug to Koller (23).
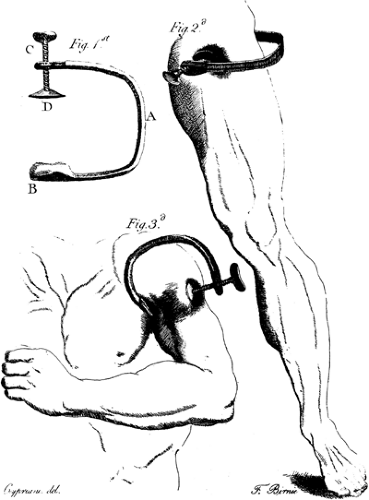 Figure 1-3. James Moore’s compression instruments for upper and lower extremities. From Moore J. A method of preventing or diminishing pain in several operations of surgery. London: T. Cadell, 1784. |
 Figure 1-6. Patrick Wall (left) and Ronald Melzack (right) receiving awards at 1989 annual meeting of the American Society of Regional Anesthesia. |
Freud wanted to know more about the analeptic action of cocaine, which, he hoped, because of reports from the United States, might be useful in curing one of his great friends of addiction to morphine. This friend was a pathologist who had developed an unbearably painful thenar neuroma after accidentally cutting himself while performing an autopsy. Freud obtained a supply of cocaine from the manufacturing firm of Merck and shared it with Koller, who was to help him investigate its effects on the nervous system. Koller was a junior intern in the Ophthalmological Clinic at the University of Vienna and longed to obtain the coveted appointment of assistant in the clinic, on the strength of a worthy piece of research. In 1884, Koller met Dr. Joseph Gartner at Stricker’s Institute for Pathological Anatomy. They dissolved a trace of the white coca powder in distilled water and instilled it in the conjunctival sac of a frog, which allowed its cornea to be touched with no evidence of reflex action or defense. Identical tests were performed on a rabbit and a dog, and the results were equally favorable. Koller wrote: “One more step had now to be taken. We trickled the solution under each other’s lifted eyelids, then placed a mirror before us, took pins, and with the head tried to touch the cornea. Almost simultaneously we were able to state jubilantly ‘I can’t feel anything (20,23).’” After these experiments, he then performed an operation for glaucoma using topical cocaine anesthesia on September 11, 1884, just 4 days before the Congress of Ophthalmology was due to meet in Heidelberg. Koller immediately wrote a paper for the Congress, but, being an impecunious intern, he could not afford the train fare to Heidelberg, so he gave the paper to a visiting ophthalmologist from Trieste, Dr. Brettauer, who had stopped in Vienna on his way to the Congress. Brettauer’s news from Heidelberg reached New York in a letter from H. D. Noyes, an American ophthalmologist who had attended the Heidelberg Congress (24).
Noyes’s letter to the New York Medical Record excited numerous readers to test the new wonder drug, and many of them rushed into print with astounding experiences. One of the most striking, published within 5 weeks of Noyes’s communication, was that of N. J. Hepburn, a New York ophthalmologist (25). There were no standards for drug trials in those days, and the tradition of self-experimentation was inviolate. If a researcher or physician wanted to know whether a drug was safe, he tried it on himself. Hepburn describes how, on October 16, 1884, he experimented with a 2% solution of cocaine, giving himself a series of subcutaneous injections of 0.4 mL (8 mg) at intervals of 5 minutes. He noted that, by the time of the eighth injection, the agreeable stimulating effects of the drug—rapid respiration and pulse, a feeling of warmth, pleasant hallucinations—had reached such a point that he felt it best to stop. For reasons that Hepburn does not state, he repeated the performance 2 days later, and then found it possible to carry the number of 0.4 mL injections to 16 before the general disturbance persuaded him to cease. He records that 4 days later, he was at it again, and this time he tried a larger unit volume and amount (10 mg), and was able to tolerate 16 of these doses. It seems likely that Hepburn was already in the grip of addiction.
By November 29, 1884, the ophthalmologist Bull was able to report that he had used the drug to produce anesthesia of the
cornea and conjunctiva in more than 150 cases (26). He gave sound reasons for his enthusiasm: He saved the time required for complete etherization and avoided the enormous engorgement of the ocular blood vessels produced by the ether, the danger of vomiting, and the disadvantage that almost any apparatus for producing anesthesia by inhalation was a physical interference for the operator.
cornea and conjunctiva in more than 150 cases (26). He gave sound reasons for his enthusiasm: He saved the time required for complete etherization and avoided the enormous engorgement of the ocular blood vessels produced by the ether, the danger of vomiting, and the disadvantage that almost any apparatus for producing anesthesia by inhalation was a physical interference for the operator.
One evening in January 1885, while he was on duty in the emergency room, a workman with an injured finger was brought in. Koller noticed there was a tourniquet applied to the base of the finger. Zinner, Billroth’s intern, asked Koller to admit the man to Billroth’s service and Koller did so, but he himself urgently removed the tourniquet in order to save the finger. This act aroused Zinner’s ire. Zinner called Koller an impudent Jew. Koller in return slapped Zinner’s face. Zinner thereupon challenged Koller to a duel. Billroth specified that all duels were strictly prohibited, but both parties were officers in the reserve and members of the Patriotic German Student Society, whose unwritten code dictated that honor must be avenged. The duel was fought with swords the next day, and Koller wounded his opponent. The law impartially charged both parties with the crime. Koller received an official pardon a few months later, but his prospects for advancement in German-speaking Europe were wrecked, destroyed by the first and last duel known to have been fought over a tourniquet. Soon, he immigrated to The Netherlands, and, with Freud’s and others’ advice, in 1885, to the United States (27). This series of events seems to be in character for Koller, whose own daughter said he was “a difficult tempestuous young man, one who could never be compelled to speak diplomatically even for his own good (28).” Following these earlier events, Koller immigrated to the United States, and he established an ophthalmology practice in New York City.
Conduction Anesthesia
After the publication of Noyes’s 1884 letter, the idea of injecting cocaine directly into tissues to render them insensible occurred simultaneously to several American surgeons. William C. Burke injected 5 minims (drops) of 2% solution close to a metacarpal branch of the ulnar nerve and painlessly extracted a 22-caliber bullet from the base of his patient’s little finger (29). But it was William Stewart Halsted (1852–1922) and Richard John Hall (1856–1897) and their associates who most clearly saw the great possibilities of conduction block (Fig. 1-9) (30,31,32). The term was introduced by François-Franck in 1892 (33), although he may well have borrowed part of it from Corning, for in 1886, Corning was writing that “the thought of producing anaesthesia by abolishing conduction in sensory nerves, by suitable means, should have been rife in the minds of progressive physicians (12).” Corning himself quite possibly got the idea from Halsted, for Halsted later attested that Corning was a frequent observer at the Roosevelt Hospital in New York, where Halsted, assisted by Hall, performed his teaching. In 1884, Hall described how he blocked a cutaneous branch of the ulnar nerve in his own forearm (34). He and Halsted made injections into the musculocutaneous nerve of the leg and the ulnar nerve. Hall noted the appearance of marked constitutional symptoms, giddiness, severe nausea, cold perspiration, and dilated pupils, but this did not daunt these bold pioneers, and that same evening Halsted blocked Hall’s supratrochlear nerve and removed an adjoining congenital cystic tumor. He also induced Nash, a dental surgeon, to tend to Hall’s own upper incisor tooth after injection of cocaine into the infraorbital nerve at the infraorbital foramen, and Halsted thereafter performed an inferior dental nerve block on a medical student volunteer and later did the same to Hall. Hall’s report was quite explicit in predicting that, once the limits of safety had been determined, this mode of administration would find very wide application in the outpatient department.
The daring experimenters at the Roosevelt Hospital unfortunately became addicted to the new drug, and no more was heard from them about its use in surgery. It appears that Dr. Halsted, with the help of his friend, Dr. William H. Welch, was the only one of the group able to overcome the addiction. In 1886, Halsted, upon an invitation from Dr. Welch, moved to Baltimore. In 1889, after his final recovery from cocaine addiction, he was appointed acting surgeon and head of the outpatient department of the newly established Johns Hopkins Hospital and, in 1890, he became professor of surgery at the new Johns Hopkins Medical School (35). But that Hall and Halsted were the true progenitors of conduction anesthesia can hardly be doubted (34,36).
The great advantage of local anesthesia with cocaine was, of course, that it anesthetized only the part of the body on which the operation was to be performed. However, a price was paid in toxicity and time. Rapid absorption limited the safe quantity of cocaine to 30 mg and the useful duration of anesthesia to 10 to 15 minutes. In 1885, Corning sought a means of prolonging the local anesthetic effects for surgical and other purposes, although he was primarily interested in the application of the drug to the therapeutics of neurologic disease (37). His notion of pharmacokinetics was that, after the introduction of cocaine beneath the skin, a certain period of time elapsed during which the anesthetic agent was diffused throughout the surrounding tissue, with the capillary circulation having a dual effect, first as a distributor and afterward as a dilutor and rapid remover
of the anesthetic substance. In his first article of 1885, Corning described how he experimentally injected 0.3 mL of a 4% solution of cocaine into the lateral antebrachial nerve and obtained immediate anesthesia of the skin supplied by this nerve as far as the wrist. He found that simple arrest of the circulation in the involved part by compression or constriction proximal to the point of injection intensified the anesthesia and prolonged it indefinitely. He used an Esmarch bandage for this purpose and pointed out that the method was readily applicable to surgery of all the extremities. The Riva-Rocci cuff tourniquet had not yet been invented. Esmarch had introduced his elastic bandage in 1874, for the purpose of producing a bloodless field in major amputations (38).
of the anesthetic substance. In his first article of 1885, Corning described how he experimentally injected 0.3 mL of a 4% solution of cocaine into the lateral antebrachial nerve and obtained immediate anesthesia of the skin supplied by this nerve as far as the wrist. He found that simple arrest of the circulation in the involved part by compression or constriction proximal to the point of injection intensified the anesthesia and prolonged it indefinitely. He used an Esmarch bandage for this purpose and pointed out that the method was readily applicable to surgery of all the extremities. The Riva-Rocci cuff tourniquet had not yet been invented. Esmarch had introduced his elastic bandage in 1874, for the purpose of producing a bloodless field in major amputations (38).
As has briefly been mentioned, François-Franck was the first to apply the term nerve blocking to the infiltration of a nerve trunk in any part (33,36). He found that the effect of the blocking drug was not limited to the purely sensory fibers because it paralyzed all nerves, whether motor or sensory, and that the sensory anesthesia was manifested much more promptly than was the motor paralysis, a confirmation of von Anrep’s observations of 1879 to 1880. François-Franck spoke of the action of cocaine as a “physiological section,” transitory and noninjurious.
Corning’s principle of prolonging the local anesthetic action of cocaine by arresting the circulation in the anesthetized area inspired Heinrich F. W. Braun (1862–1934) to dispense with the elastic tourniquet and substitute epinephrine, a “chemical tourniquet” as he called it (39). Epinephrine had become available in pure form after Abel isolated it from the suprarenal medulla in 1897 (40).
The suggestion for this use of epinephrine came from ophthalmologic practice, in which it had been introduced to limit hemorrhage and to render the conjunctiva bloodless, as well as to treat certain diseases, notably glaucoma, in which it was found to prolong the local effect of other drugs in general and of cocaine in particular. This observation had been confirmed by rhinologists and had enabled them to reduce the concentration and dose of cocaine and correspondingly to limit the hazard of toxicity (41). Initially, in Braun’s solution, the epinephrine was present in concentrations from 1:10,000 to 1:100,000. The first experiments to determine the dosage to be injected subcutaneously were made by Braun on himself. He found his limit of tolerance was 0.5 mg (0.5 mL of 1:1000 solution), after which general symptoms occurred, and he had to lie down.
Braun introduced the term conduction anesthesia, and he felt that the use of epinephrine rendered conduction anesthesia in other parts of the body as effective as that in an extremity. In 1905, Braun published a textbook on local anesthesia, giving detailed descriptions of the technique for every region (Table 1-2).
Infiltration Anesthesia
Some 10 years earlier, a different approach, termed infiltration anesthesia, had been advocated by Karl Ludwig Schleich (1859–1922) (42). Schleich’s interest in infiltration anesthesia appeared to stem from the poor effects that often followed general anesthesia in that era. Schleich stated: “For however great the improvement that our methods in the treatment of narcosis may undergo in the course of time, it will always remain a dangerous and uncertain interference with the brain mechanism, the working of which we are unable to fathom … What a blessing, then, if narcosis can be avoided in so great a proportion of cases (35,42)!” Schleich applied the principle that pure water has a weak anesthetic effect but is painful on injection, whereas physiologic saline is not. Although Schleich’s initial report on infiltration anesthesia before the Congress of Surgeons in Mainz, Germany, was unfavorably received, by the early 20th century, this technique of anesthesia was widely used. It is reported that Schleich was a meticulous technician who gave great attention to detail, and this likely explains his success over time (35).
The observation that subcutaneous injection of water produced local anesthesia was apparently first made by Potain in 1869. Halsted, in a short letter to the editor of the New York Medical Journal, dated September 19, 1885 (31), baldly asserted that “the skin can be completely anesthetized to any extent by cutaneous injections of water;” he had of late used water instead of cocaine in skin incisions, and the anesthesia did not always vanish just as soon as hyperemia supervened.
Schleich believed that there must be a solution of such a concentration between “normal” (0.6% salt solution) and pure water that would not provoke pain on injection and yet be usefully anesthetic, and he thought a 0.2% solution of sodium chloride was ideal. To this, he added cocaine to a concentration of 0.02% and employed the mixture to produce a field of cutaneous anesthesia in the surgery of hydrocele, sebaceous cyst, hemorrhoids, and small abscesses.
The reason why Schleich’s hypotonic solutions containing a minuscule amount of cocaine produced impairment of sensation does not appear to have been explained. In the light of later work, it seems possible that loss of electrolyte from nerve fibers may have been involved. Braun dismissed Schleich’s solutions as nonphysiologic and insisted that injections into the tissues for whatever purpose must be composed of fluids of the same osmotic tension as the body fluids. Inasmuch as most local anesthetic solutions are hypotonic, a corresponding amount of an indifferent salt, such as sodium chloride, must be added to prevent any injurious action upon the tissue.
Nevertheless, Schleich’s infiltration technique was an important advance in that it extended the field of usefulness of a small quantity of anesthetic. Schleich was probably indebted to Paul Reclus for the idea of using a weak solution of cocaine to avoid toxic reactions and fatalities. Enthusiasm for local anesthesia had diminished owing to casualties, but Reclus clearly understood that the basic cause of accidental deaths was overdose from the use of unnecessarily high concentrations (43). He realized that undue absorption could be avoided by using lower concentrations, and he eventually reduced the strength of his cocaine solutions to 0.5%.
Local Anesthetics
The toxicity of cocaine, coupled with its vast potential for usefulness in surgery, led to an intensive search for less toxic substitutes. However, decreased toxicity without increased irritancy—or impractically brief effectiveness—proved elusive until the synthesis of procaine (Novocaine) by Einhorn in 1904. No specific report to that effect appears in the literature, so it fell to the lot of the surgeon Heinrich Braun to make the report in 1905, along with descriptions of two other agents, stovaine and alypin (44). Procaine’s short duration of activity limited clinical utility; thus, research focused on dibucaine (1925). Meischer synthesized dibucaine, a quinoline derivative, which Uhlmann introduced clinically. In 1928, Eisleb synthesized tetracaine, which was then introduced into clinical practice in 1932. Although dibucaine and tetracaine proved to be potent, long-acting local anesthetics, their increased systemic toxicity limited the usefulness of these agents for many of the regional anesthetic techniques in which large volumes of drugs
were required. Thus, these agents found their primary use in the field of spinal anesthesia, and they continue to be used today. Most of the chemical compounds synthesized during this first pharmaceutical period were amino ester derivatives, similar in most respects to cocaine. Most of these amino ester agents were relatively unstable and could not be subjected to repeated autoclaving for sterilization. In addition, the hydrolysis of amino esters by the enzyme plasma pseudocholinesterase resulted in the formation of para-aminobenzoic acid, which was responsible for reported allergic reactions (45). Additional crucial properties for wider use of local anesthetics—namely, chemical stability and absence of sensitization—were achieved with lidocaine, which was introduced in 1947 (Table 1-3) (46).
were required. Thus, these agents found their primary use in the field of spinal anesthesia, and they continue to be used today. Most of the chemical compounds synthesized during this first pharmaceutical period were amino ester derivatives, similar in most respects to cocaine. Most of these amino ester agents were relatively unstable and could not be subjected to repeated autoclaving for sterilization. In addition, the hydrolysis of amino esters by the enzyme plasma pseudocholinesterase resulted in the formation of para-aminobenzoic acid, which was responsible for reported allergic reactions (45). Additional crucial properties for wider use of local anesthetics—namely, chemical stability and absence of sensitization—were achieved with lidocaine, which was introduced in 1947 (Table 1-3) (46).
Table 1-3 Chronology of Local Anesthetic Agents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Lidocaine, synthesized in 1943 by Löfgren and Lundqvist, was a stable compound that was not influenced by repeated exposures to high temperatures and thus could be resterilized often. In addition, the metabolites of lidocaine did not include para-aminobenzoic acid; thus, allergic reactions were avoided.
Lundqvist, like many early investigators, started using lidocaine on his own toes and fingers and even for spinal anesthesia. In August 1943, Lundqvist called another friend—Lagergreen—and said that a friend of his had synthesized a new local anesthetic. They arranged a meeting with representatives of the drug company Pharmacia and asked if Lagergreen would come as their medical expert and demonstrate finger blocks on volunteers. This he did, performing five to ten of the blocks, and the results were demonstrated for the executives of Pharmacia. They were to be given a decision within 2 weeks, but Löfgren and Lundqvist never heard from them. Since there had been no response after the respite time, Lundqvist called Lagergreen and said that a Mr. Jordan, science attaché at the U.S. embassy, wanted to meet them. Lagergreen went with them more as an interpreter. Mr. Jordan made an immediate offer of $15,000 for the rights to the discovery, but nothing was decided. The gossip spread like wild fire, and soon Ciba, Roche, and Bayer were out to get this new wonder drug. Also, ICI was interested, and it is said that Löfgren went to London in the tail of a Mustang, a plane flying ball bearings from Sweden to England, by night during the war. However, again no decision was made. This happened between August 23 and September 10, 1943, when Astra laboratories bought the method and patent (47). At this point, the clinical testing of lidocaine was conducted by Gordh (Fig. 1-10) with the assistance of his wife, Ulla, also a physician. Volunteer patients were given 5 crowns (60 cents) for their help. Students were given a copy of Gordh’s thesis (1945) or a package of American cigarettes, which were very difficult to obtain during the war. Most of them chose the cigarettes. Gordh made his first presentation of clinical results in 1947, at a meeting of the Swedish Anesthesia Club, the predecessor of the Swedish Society of Anesthesiologists. In the same year, his paper was also presented at the Scandinavian Surgical Society’s first meeting after the war. The results were published in Svenskja Lakartidningen in 1948 (47).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree