1. Awareness and management of intraocular pressure (IOP) is an important part of the anesthetic care of children undergoing many ophthalmologic procedures.
2. In most ophthalmologic surgeries, the patient must be kept immobile. In particular, movement of the extraocular muscles often must be controlled.
3. It is important to understand and manage the oculocardiac and oculogastric reflexes in ophthalmologic surgery.
4. Many ophthalmologic medications have systemic effects of importance to the anesthesiologist.
5. It is very important to minimize coughing or straining during extubation to prevent increases in IOP.
BACKGROUND
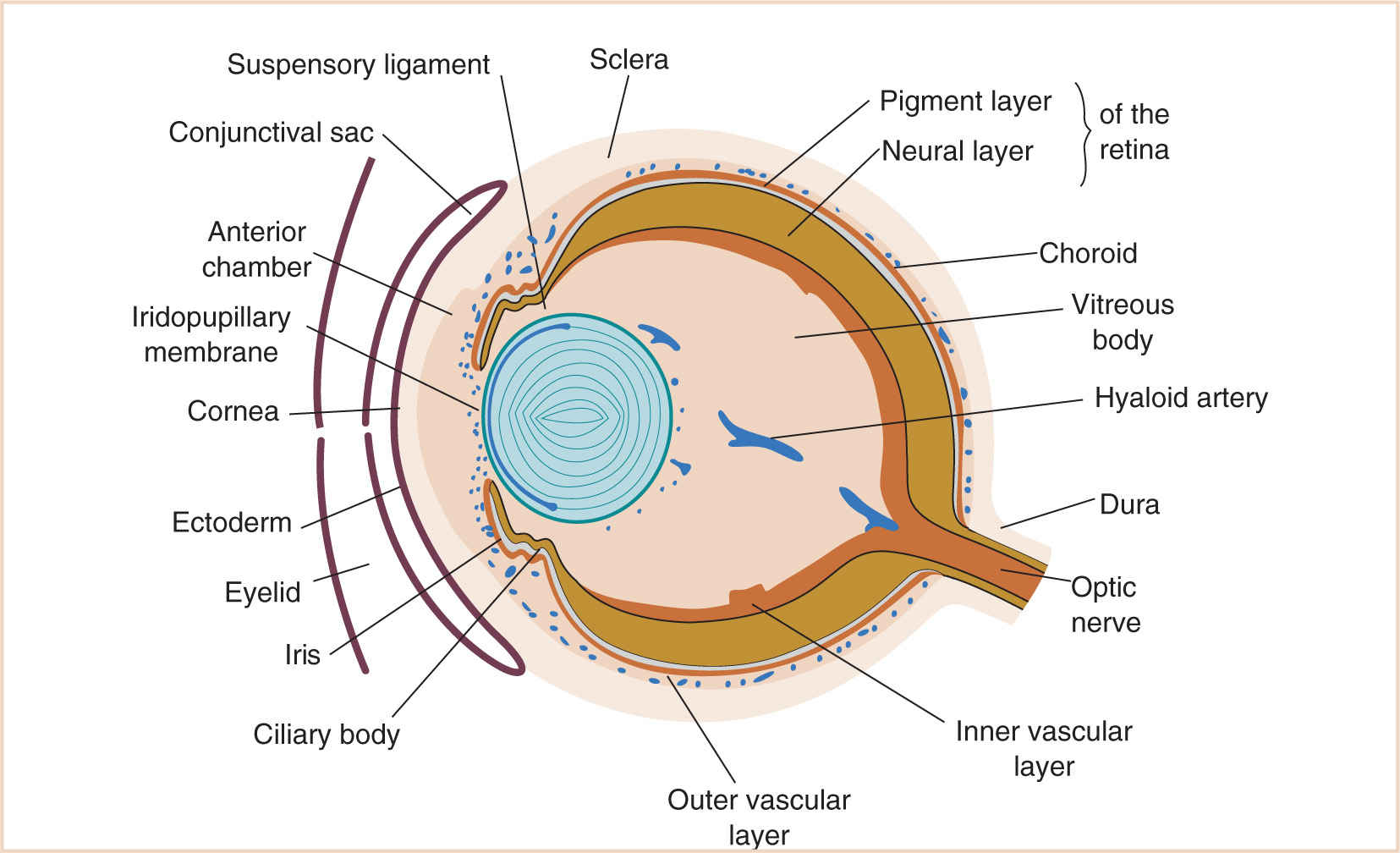
The eye develops from a complex series of genetic signaling and differentiation pathways. The final optic structures arise from three primordial zones: the surface ectoderm including the neural crest, the neural endoderm, and the mesoderm (Table 15.1). The eye develops very early in gestation, and it continues to mature after birth. Proper development of the eye relies on the integrity of signaling and differentiation pathways, but can also be influenced by exogenous factors such as many of the supportive therapies that may be used in the treatment of a preterm neonate.
TABLE 15.1 Embryological origins of eye structures
Primordial origin | Mature anatomic structure of the eye |
Surface ectoderm Neural crest | Lens Lacrimal gland Corneal epithelium Conjunctiva Epidermis of the eyelids Corneal keratocytes Corneal endothelium Trabecular meshwork Stroma of iris and choroid Ciliary muscle Fibroblasts of sclera Orbital cartilage and bones Orbital connective tissue Extraocular muscles Subepidermal layers of the eye |
Neural ectoderm | Retina Retinal pigmented epithelium (RPE) Ciliary epithelium Dilatory muscles of the iris Optic nerve fibers Glial cells |
Mesoderm | Extraocular muscles Orbit and ocular vascular endothelium |
Children of varying age and health status may present for ophthalmologic surgical procedures. Their diagnosis may be due to a developmental or acquired abnormality. They may also have an associated syndrome with other systemic considerations for the anesthesiologist.
EMBRYOLOGY/ANATOMY
At 3 weeks of development (optic vesicle stage), the most cephalic portion of the neural tube develops mirrored outpouchings (optic grooves). These sulcus/groove structures of neural ectoderm advance outward through the mesoderm toward the surface ectoderm. Meanwhile, the overriding surface ectoderm tissue becomes thicker, differentiating into a lens plate (1) (Fig. 15.1).
As the optic vesicle continues to expand outward in the 4th week, the surface ectoderm over it thickens, forming the lens placode. The vesicle invaginates and folds on itself, creating a cup-like shape. The concave aspect of this cup engulfs the lens plate mesenchyme. The inner surface of this optic cup is the precursor to the neural retina, while the thinner outer cup layer will develop into the retinal pigmented epithelium (RPE) (2).
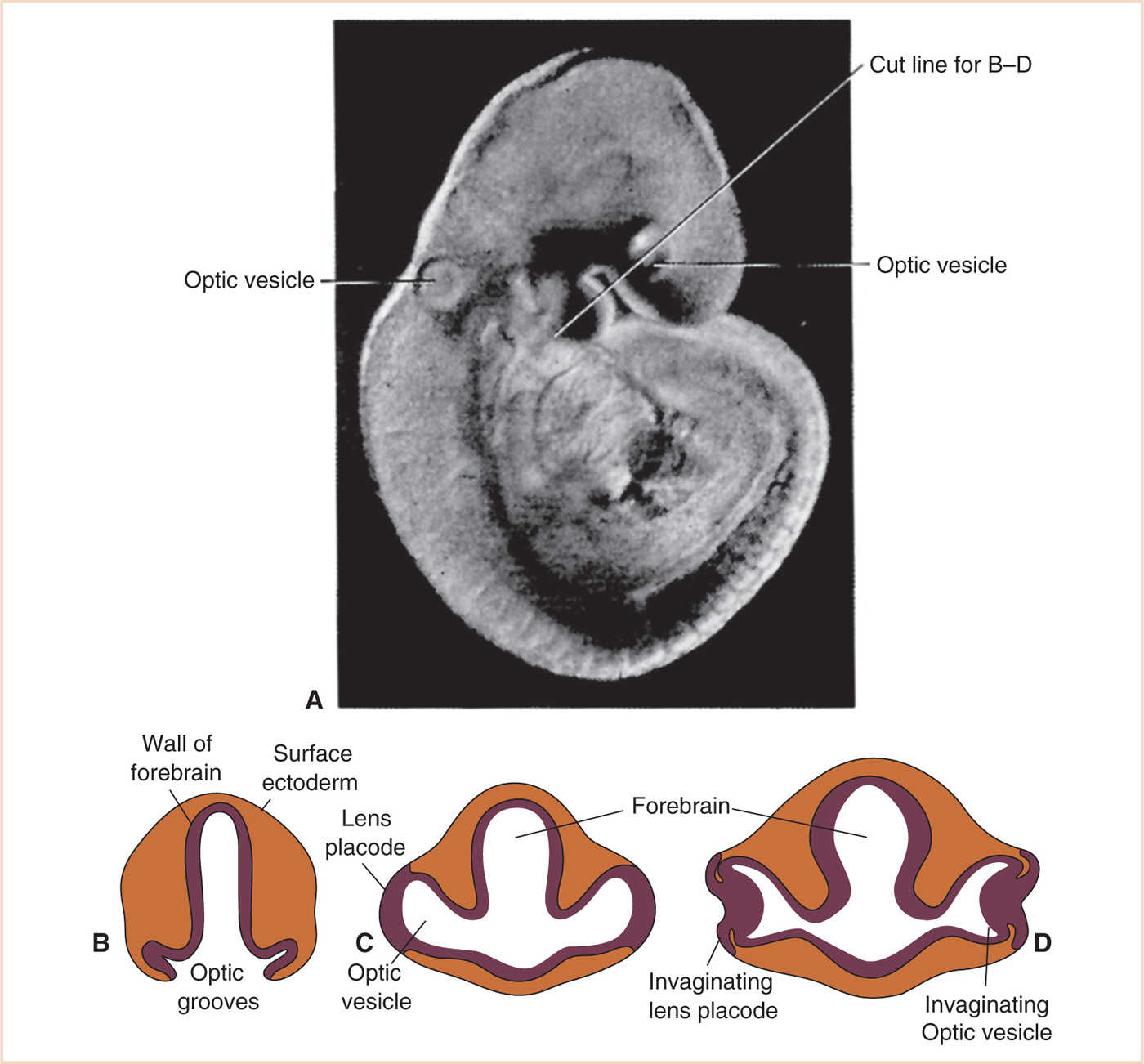
Figure 15.1 Optic vesicle stage (3). A: Embryo at 4 weeks. B: Forebrain transverse section at 3 weeks. C: Transverse section through forebrain at 4 weeks. D: Embryo transitioning from optic vesicle stage to optic cup stage as vesicle begins to invaginate.
At 5 to 6 weeks, the corneal epithelium and endothelium become apparent (1). The undersurface of the optic cup, the choroid fissure, is initially open from the original outward expansion from the neural tube. This fissure communicates with the ventricular system of the developing brain. It closes when the sides meet, forming the optic stalk, in which the hyaloid artery exists (1,3), the precursor to the retinal artery. At 7 weeks, the optic stalk develops into the optic nerve as nerve sprouts extend back from the developing neural retina to the thalamus and myelination begins in a central to peripheral direction (1). Also forming at this stage are the sclerae and extraocular muscles (Fig. 15.2).
At 8 weeks, the lids have grown out over the surface of the eye and actually fuse over the developing globe. Branches of the highly arborized hyaloid vasculature anastomose around the optic cup margin creating the ciliary artery system. The vessels then regress, creating an avascular lens and vitreous necessary for light transmission (Fig. 15.3). At 10 weeks, suspensory ligaments for the lens also develop from the rudimentary vitreous vessels now turned to vitreous fibers, anchoring the lens to the ciliary epithelium. There now is a separation between the vitreous and the anterior/posterior chambers of the eye. Schlemm’s canal forms. At the 3rd month of development, the optic cup rim extends into the anterior chamber, creating the iris (4). The basic structures and chambers of the eye are now determined. As the iris and the structures of the anterior chamber grow and develop, Schlemm’s canal location changes with regard to the anterior chamber angle.
At month 4 to 5, the retinal artery replaces the hyaloid artery to supply the retina. Anteriorly, the cornea and sclera are differentiated (1). The fused lids separate. The insertions of the rectus muscles are completed. At month 6, pupillary dilation muscles appear in the ciliary body with norepinephrine-responsive action. Later, the acetylcholine-sensitive pupillary constrictors arise from the inner neural retinal layer.
At month 7, secondary lens fibers that grow posterior to the lens capsular epithelium are present and form the y-sutures of the lens. In the retina, the outer rod and cone nuclei form and also present are the nerve fibers, bipolar cells, amacrine, and ganglion cells. At month 8, the macular area of the retina is present.
After birth, myelination of the optic nerve continues from central to peripheral and is complete by the 3rd postnatal month. Nasolacrimal duct canals develop just before birth and during the first postnatal month (1). The corneal reflex is not reliably present in infants until 3 months of age (5). The anterior-to-posterior dimension of the eye is significantly foreshortened compared to the adult eye, but the more bulbous shape of the lens at birth compensates for this by providing a higher degree of refraction. As the eye shape elongates and the lens flattens, the final optics of the eye are defined, which occurs around 7 or 8 years of age (1).
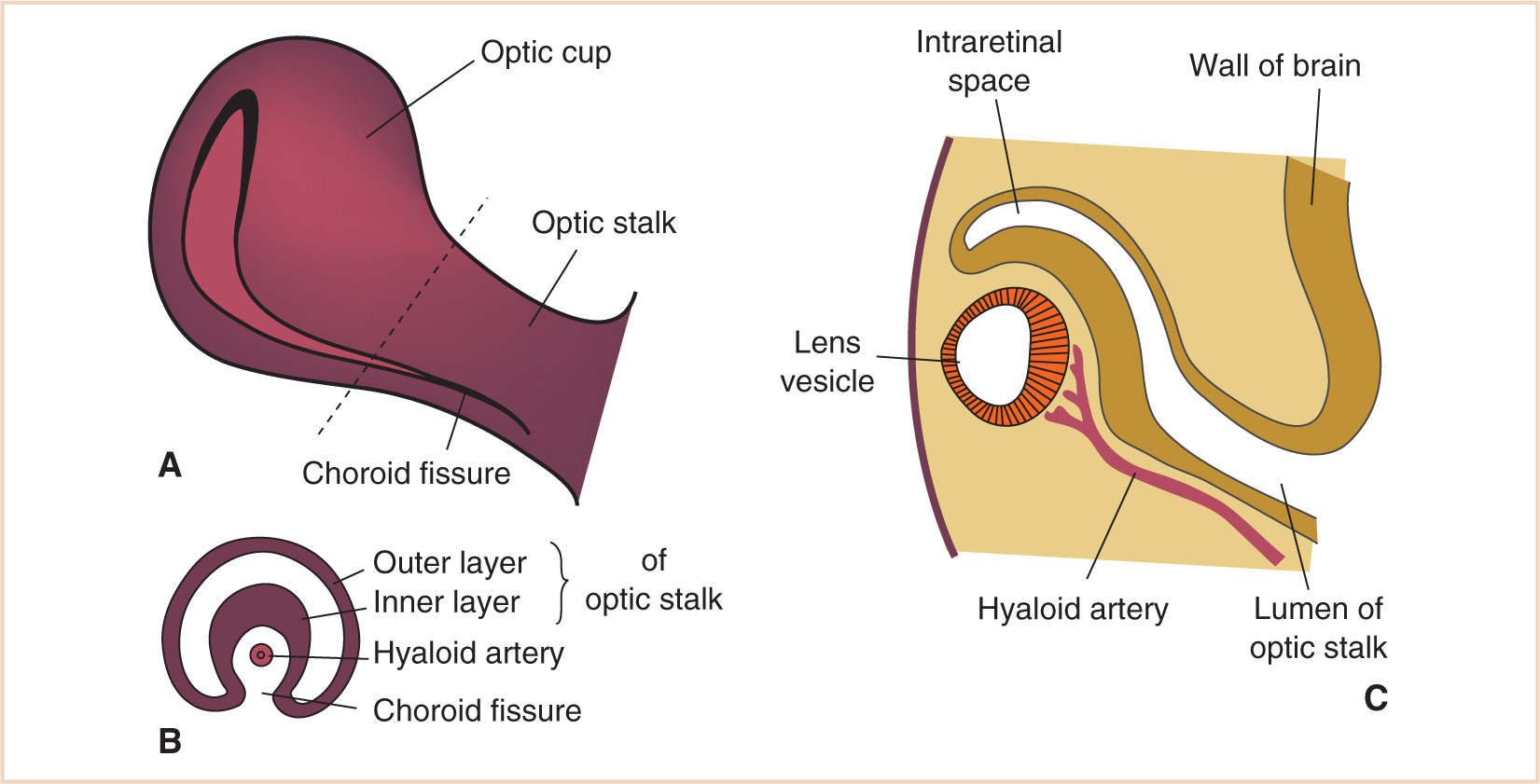
Figure 15.2 Optic cup stage (3). A: Optic cup formed. B: Transverse section of the optic stalk at the hashed line of (A). C: Section through the optic cup along the choroid fissure.
PHYSIOLOGIC CONSIDERATIONS
INTRAOCULAR SURGERY
1. IOP
2. Extraocular muscle tone
3. Extraocular surgery or examinations:
a. Oculocardiac reflex. This reflex is more dramatic and occurs more frequently in pediatric patients owing to their higher vagal tone with respect to the sympathetic system development (6).
b. Oculogastric reflex causing high incidence of postoperative nausea and vomiting
4. Infection can be localized to the periorbital structures, or the patient may be septic with relative intravascular volume depletion.
5. Systemic effects of ophthalmic medications
6. Metabolic disorders involving dysregulation of calcium or glucose
7. See Table 15.2
a. Congenital heart diseases
b. Renal disease
c. Coagulopathies
d. Dysmorphic facial features with possible difficult intubation
e. Developmental delay
8. Prematurity
9. Side effects of concomitant therapies
10. Steroid induced diabetes
11. Cardiopulmonary disease from chemotherapy
SURGICAL REPAIRS
EXTRAOCULAR
1. Evaluation under anesthesia (EUA): Infants and small children usually require a general anesthetic for a comprehensive ophthalmic examination.
TABLE 15.2 Ophthalmologic disorders associated with syndromes
Ocular abnormality | Frequently associated syndromes |
Glaucoma: overproduction or lack of resorption of aqueous humor, causing increased IOP and sometimes a cloudy cornea | Sturge Weber syndrome Neurofibromatosis Marfan syndrome Pierre Robin Homocystinuria Lowe syndrome (oculocerebrorenal dystrophy) Rubinstein Taybi |
Anopthalmos: missing portions or all tissues of the eye | Klinefelter Trisomy 13 Goldenhar (oculoauricular dysplasia) |
Coloboma: missing tissue of the iris therein giving a notched appearance to the pupil | CHARGE syndrome |
Corneal opacities | Goldenhar syndrome |
Aniridia: lack of iris development | Wilms tumor |
Cataracts: idiopathic or syndrome associated opacity of the lens | Stickler syndrome Lowe syndrome Trisomy 21 Lawrence Moon Biedl Marfan syndrome |
Lens dislocation | Marfan syndrome |
Cloudy cornea | Peter anomaly |
| Hurler syndrome |
Uveitis | Spondyloarthropathies Juvenile rheumatoid arthritis |
Retinal hemorrhages | Shaken baby syndrome |
Strabismus | Cerebral palsy |
2. Electroretinography (ERG): Measurement of the retinal responses to generated light flashes. This examination requires several minutes of dark adaptation and then darkness during the procedure. Electro-oculography (EOG) measures corneoretinal potentials. Visual evoked responses (VER) measure electrical responses in the cortex to visual stimuli, but require a completely intact visual pathway as the sensory electrodes are on the scalp (1).
3. Trauma surgery: Surgical repair begins with a comprehensive EUA; subsequent treatment is aimed at minimizing infection and preserving vision.
4. Strabismus surgery: It can be uni- or bilateral and performed on single or multiple muscles. Muscle lengths (resection) or insertion sites (recession) are manipulated for better eye alignment. Often, adjustable sutures are placed in the operative eye muscle, which can later be tightened or loosened to make final adjustments to the alignment.
5. Congenital ptosis correction: Repairs are aimed at elevating the lid off the eye to prevent deprivation amblyopia.
6. Cysts drainage: Cysts from the sweat glands of the eye lid or in conjunction with the sweat glands of the eyelashes may require surgical drainage.
7. Lacrimal duct probing: This is usually a very quick procedure, less than 5 minutes per eye, which explores the patency of each duct. It can be curative just by probing the ducts, but sometimes a stent is needed.
8. Incision and drainage: Surgical incision and drainage of an infected orbit and/or nearby sinuses may be necessary if antibiotic therapy is ineffective.
INTRAOCULAR
1. Cataract removal: Microscopic removal of lens via a small incision in the cornea that is then sutured closed.
2. Glaucoma surgery: When IOP is ≥22 mm Hg, the pressure in the globe puts perfusion of the retina at risk. This may be due to a sclerosed trabecular network as in the case of open angle glaucoma, or a mechanical blockage by the iris, which when too close to the corneal angle can block aqueous drainage. Surgical repair is aimed at opening or creating new drainage for the aqueous humor via trabeculotomy into Schlemm’s canal, or by decreasing aqueous production with techniques such as cryotherapy of the ciliary body (7).
3. Retinoblastoma resection: Treated with repeated radiation therapy sessions or possibly enucleation of the globe and involved periorbital structures. Treatment may also include chemotherapy.
4. Retinopathy of prematurity (ROP) repair: Pending the stage (location where vascularization has halted in its developing central to peripheral course) and class (acute changes that lead to complete retinal detachment) of the disease (8), surgical technique will vary. For destruction of peripheral retinal areas to discourage neovascularization, cryoablation or laser may be used. If retinal detachment has occurred, surgeons may utilize pneumatic retinopexy whereby an expanding gas (i.e., sulfur hexafluoride or perfluoropropane) or a silicone oil is used to stop further detachment by a manner of tamponade (9). Alternatively, a scleral buckle procedure is used, where a band is placed exteriorly around the globe, which diminishes the vitreal traction on the detached retina. During the repair, a vitrectomy, which replaces vitreous humor with saline, may be needed as well.
ANESTHESIA ISSUES
READINESS FOR SURGERY
1. Open globe trauma is a true emergency.
2. Full preoperative evaluation and appropriate NPO status for elective surgery.
3. Many eye procedures can be accomplished on an outpatient basis. Ex-preterm babies less than 55 weeks postconceptual age have an increased risk of apnea after undergoing general anesthesia and extended (overnight) monitoring is indicated for these infants.
ANESTHESIA GOALS
1. Provide an immobile patient with a secured airway in most situations, as the head of the patient may not be readily accessible once the patient is positioned and the table turned.
2. Avoid increases in IOP especially in an open globe trauma but also during intraocular procedures
a. Extraocular compression (contraction of rectus muscles, extrinsic pressure)
b. Change in intraocular contents (increased blood flow or decreased venous drainage)
3. Analgesia
a. Topical anesthetics by the ophthalmologist or retrobulbar block
b. Systemic opioids and acetaminophen are frequently used. Nonsteroidal anti-inflammatory drugs can be utilized for extraocular procedures, but are often avoided in cases with intraocular bleeding risks.
4. Minimizing or avoiding the oculocardiac reflex
a. Discontinuing surgical manipulation
b. Treatment (or pretreatment) with a vagolytic such as atropine or glycopyrrolate
5. Minimize bleeding
a. Avoid hypertension
b. Smooth emergence
c. Good postoperative analgesia
d. Minimizing postoperative nausea and vomiting
6. Awareness of ophthalmic eyedrop medications and their potential systemic effects (frequently used: epinephrine, acetylcholine, anticholinesterases, cocaine, cyclopentolate, phenylephrine, and ß blockers (9)).
GENERAL ANESTHESIA
Position: Supine with head at the top edge of the bed on a headrest and immediately below a microscope. The patient is typically rotated away from the anesthesiologist by at least 90 degrees to allow surgical access.
Typical Surgical Time: EUAs and lacrimal probing may be only 15 minutes, but more complex repairs may take 2 to 4 hours.
Induction
CLINICAL PEARL Children with ophthalmologic trauma for repair of an open globe present a significant challenge for the anesthesiologist. These patients are generally not NPO for an appropriate amount of time and also, as a result of trauma, have delayed gastric emptying. However, if an Rapid Sequence Intubation (RSI) is not performed with exquisite care, it is possible that increases in IOP could result in extrusion of vitreous humor with potential loss of vision.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree