FIG. 11.1 Events of cardiac cycle showing changes in left atrial pressure, left ventricular pressure, aortic pressure, ventricular volume, electrocardiogram, and phonocardiogram. A-V, Atrioventricular. (From Hall JE: Guyton and Hall textbook of medical physiology, ed 13, Philadelphia, PA, 2016, Elsevier.)
Cardiac Output
Cardiac output is the amount of blood ejected from the left or right ventricle in 1 minute. In the normal adult with a heart rate of 70 bpm, the cardiac output is approximately 5000 to 6000 mL/minute. This estimate can be derived by taking the rate of 70 bpm times the stroke volume of 70 mL. User-friendly sophisticated equipment is available to monitor a patient’s cardiac output in the PACU. The information derived from serial measurements of the cardiac output can be helpful in assessing the general status of the cardiovascular system and determining the appropriate amount and type of fluid therapy for the patient.5–6
The cardiac output is measured via a variety of techniques. Kaplan suggests the thermodilution method, which uses a pulmonary artery catheter, is the clinical method of choice. For a higher degree of reproducibility, Kaplan recommends a technique of standardization in which the injectate temperature and volume and the speed of injection are carefully controlled and duplicated. The most reproducible results have been obtained with injections of 10 mL of cold (1° to 2° C) 5% dextrose in water. It is important to remember that the thermodilution technique measures right-sided cardiac outputs; therefore, measurements of cardiac output with the thermodilution technique are usually unreliable for patients with intracardiac shunts.3,6,7
Other methods of calculation of the cardiac output are the Fick and Stewart techniques. The Fick technique involves calculations of the amount of blood needed to carry oxygen taken up from the alveoli per unit of time. This technique is said to be accurate within a 10% margin of error. In the Stewart technique, a known quantity of dye is injected, and its concentration is measured after the dye is dispersed per unit of time.7
Cardiac output can be influenced by venous return. If the heart receives an extra amount of blood from the veins (↑ preload), the cardiac muscle becomes stretched and the stretched muscle contracts with an increased force to pump the extra blood out of the heart. If the heart receives less blood than normal (↓ preload), according to the Frank-Starling law of the heart, it contracts with less force. This concept is important to the perianesthesia nurse. For example, if a patient is undergoing mechanical ventilation and too much positive end-expiratory pressure is overinflating the lungs, the increased pressure on the inferior vena cava impedes the venous return to the heart, thereby decreasing blood pressure. The blood pressure is derived from the following interacting factors: the force of the heart, the peripheral resistance, the volume of blood, the viscosity of blood, and the elasticity of the arteries. Thus, cardiac output can be seen to play a major role in the maintenance of a normal blood pressure.6–8
Arterial Blood Pressure
The arterial blood pressure consists of the systolic and diastolic arterial pressures. The systolic blood pressure is the highest pressure that occurs within an artery during each contraction of the heart. The diastolic blood pressure is the lowest pressure that occurs within an artery during each contraction of the heart. The mean arterial pressure is the average pressure that pushes blood through the systemic circulatory system. Methods of assessing and monitoring the arterial blood pressure in the PACU are discussed in Chapter 27.
Some factors that affect the arterial blood pressure are the vasomotor center, the renal system, vascular resistance, the endocrine system, and chemical regulation. The vasomotor center, located in the pons and the medulla, has the greatest control over the circulation. This center picks up impulses from all over the body and transmits them down the spinal cord and through vasoconstrictor fibers to most vessels of the body. These impulses can be excitatory or inhibitory. One type of pressoreceptor that sends impulses to the vasomotor center is the baroreceptor. The baroreceptors are located in the walls of the major thoracic and neck arteries, in particular the arch of the aorta. When these vessels are stretched by an increased blood pressure, they send inhibitory impulses to the vasomotor center, which lowers the blood pressure. The aortic and carotid bodies located in the bifurcation of the carotid arteries and along the aortic arch can increase systemic pressure when stimulated by a low partial pressure of oxygen in arterial blood (PaO2).9,10 The renal regulation of arterial pressure occurs through the renin-angiotensin-aldosterone mechanism (see Chapter 13).
The vascular resistance of the systemic vascular system can alter systemic pressure. As the total cross-sectional area of an artery decreases, the systemic vascular resistance increases. Therefore, as the blood flows out of the aorta, a decrease in the arterial pressure in each portion of the systemic circulation is directly proportional to the amount of vascular resistance. This principle is the reason the arterial pressure in the aorta is much higher than the pressure in the arterioles, which have a small cross-sectional area.
The nervous system, when stimulated with exercise or stress, elevates the arterial pressure via sympathetic vasoconstrictor fibers throughout the body.
Historically, when the radial artery was to be cannulated for direct monitoring of blood pressure and sampling of arterial blood gases in the PACU, a modified Allen test was performed. This test was used for assessing the risk of hand ischemia if occlusion of the cannulated vessel occurred. The modified Allen test is performed with the patient making a tight fist, which partially exsanguinates the hand. The nurse then occludes both the radial and the ulnar arteries with digital pressure. The patient is asked to open the hand, and the compressed radial artery is then released. Blushing of the palm (postischemic hyperemia) should be observed. After approximately 1 minute, the test should be repeated on the same hand with the nurse now releasing the ulnar artery while continuing to compress the radial artery. If the release of pressure over the ulnar artery does not lead to postischemic hyperemia, the contralateral artery should be similarly evaluated. The results of the modified Allen test should be reported as “refill time” for each artery. The modified Allen test is subjective at most and rarely used in clinical practice. Many clinicians are using bedside ultrasound to evaluate blood flow to the radial artery.11
Valves of the Heart
The semilunar valves are the aortic and pulmonary valves. They consist of three symmetric valve cusps, which can open to the full diameter of the ring yet provide a perfect seal when closed. During diastole, they prevent backflow from the aorta and pulmonary arteries into the ventricles.
The AV valves are the tricuspid and mitral valves. These valves prevent blood from flowing back into the atria from the ventricles during systole.
Attached to the valves are the chordae tendineae, which are attached to the papillary muscles, which in turn are attached to the endocardium of the ventricles. When the ventricles contract, so do the papillary muscles, thus pulling the valves toward the ventricles to prevent bulging of the valves into the atria (Fig. 11.2).1,2
Heart Muscle
The heart muscle comprises three major muscle types: atrial muscle, ventricular muscle, and conductive muscle fibers. The atrial and ventricular muscles act much like skeletal muscles. The conductive muscles function primarily as an excitatory system for the heart and a transmission system for conducting impulses throughout the heart.
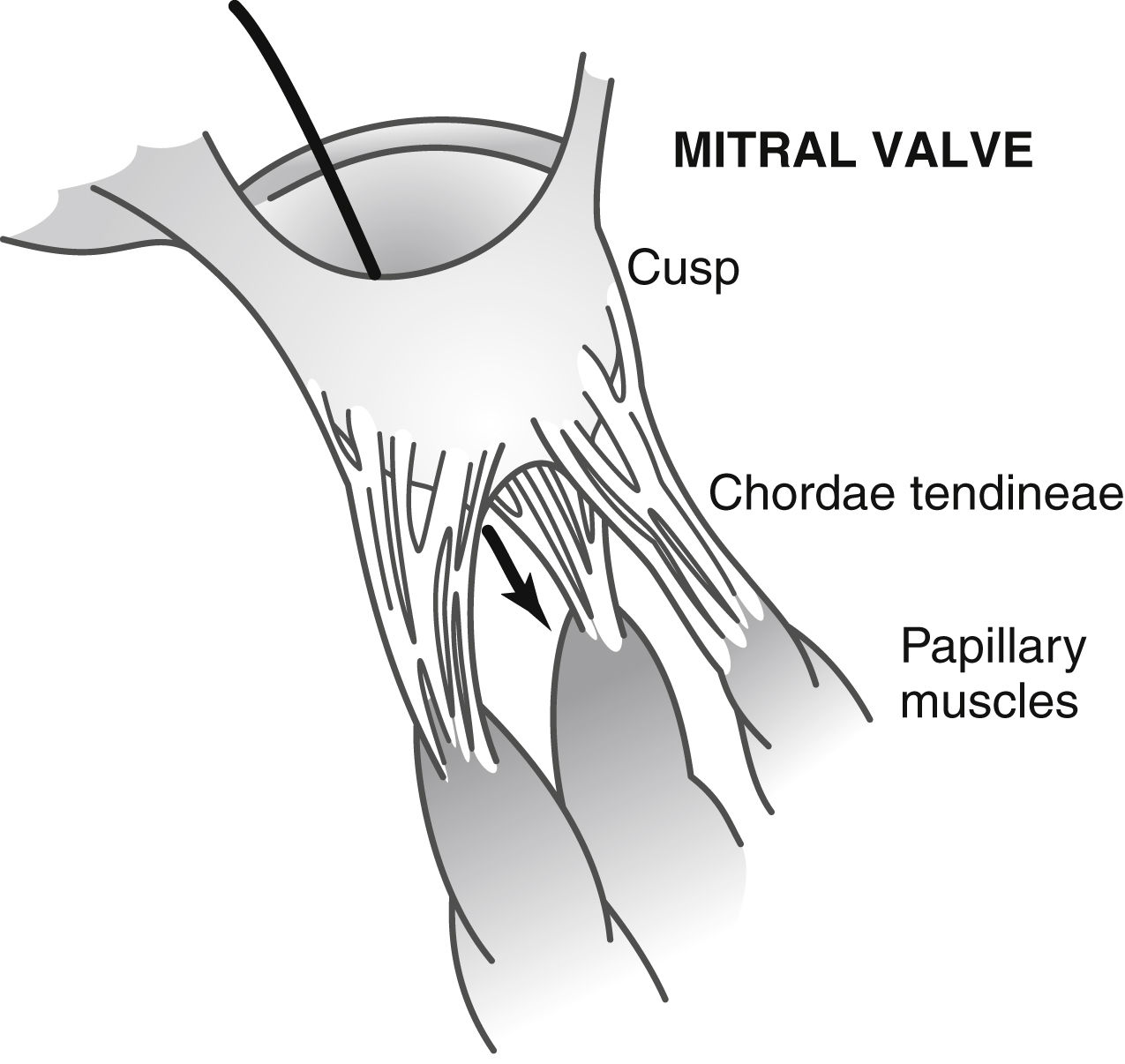
FIG. 11.2 Mitral valve and its attachments. (From Hall JE: Guyton and Hall textbook of medical physiology, ed 13, Philadelphia, PA, 2016, Elsevier.)
The cardiac muscle fibers are arranged in a latticework; they divide and then rejoin. The constriction of the cardiac muscle fibers facilitates action potential transmission. The muscle is striated, and the myofibrils contain myosin and actin filaments. Cardiac muscle cells are separated by intercalated disks, which are actually the cardiac cell membranes that separate the cardiac muscle cells from one another. The intercalated disks do not hinder conductivity or ionic transport between cardiac muscle cells to any great extent. When the cardiac muscle is stimulated, the action potential spreads to excite all the muscles, which is called a functional syncytium (Fig. 11.3). This functional syncytium can be divided into atrial and ventricular syncytia, which are separated by fibrous tissue. However, an impulse can be transmitted throughout the atrial syncytium and then via the AV bundle to the ventricular syncytium. The “all-or-none” principle is in effect; when one atrial muscle fiber is stimulated, all the atrial muscle fibers react if the action potential is met. This principle applies to the entire ventricular syncytium as well.1,2
The main properties of cardiac muscle are excitability (bathmotropism), contractility (inotropism), rhythmicity and rate (chronotropism), and conductivity (dromotropism). When cardiac muscle is excited, its action potential is reached and the muscle contracts. Certain chemical factors alter the excitability and contractility of cardiac muscle (Box 11.1).
Conduction of Impulses
The heart has a special system for generating rhythmic impulses. This system for providing rhythmicity and conductivity consists of the sinoatrial (SA) node, the AV node, the AV bundle, and the Purkinje fibers (Fig. 11.4). The SA node is situated at the posterior wall of the right atrium just below the opening of the superior vena cava. The SA node generates impulses with self-excitation and is the fastest pacemaker of the heart. It is produced by the interaction of sodium and potassium ions. The SA node provides a rhythmic excitation approximately 72 times per minute in an adult at rest. The action potential then spreads throughout the atria to the AV node via several tracts and has been implicated in abnormal atrial rhythms such as atrial flutter and fibrillation.1,2
The AV node is located at the base of the wall between the atria. Its primary function is to delay the transmission of the impulses to the ventricles, which allows time for the atria to empty before the ventricles contract. The impulses then travel through the AV bundle, sometimes called the bundle of His. The AV node is able to discharge impulses 40 to 60 times per minute if not stimulated by an outside source.
The Purkinje fibers originate at the AV node, form the AV bundle, divide into the right and left bundle branches, and spread downward around the ventricles. The Purkinje fibers can transmit the action potential rapidly, thus allowing immediate transmission of the cardiac impulse throughout the ventricles. The Purkinje fibers are able to discharge impulses between 15 and 40 times per minute if not stimulated by an outside source. The Purkinje fibers have been implicated in right and left bundle branch conduction delays, which can lead to slower blood supply to the ventricles.
The parasympathetic nerve endings are distributed mostly at the SA and AV nodes, over the atria, and to a lesser extent over the ventricles. If stimulated, they produce a decrease in the rate of rhythm of the SA node and slow the excitability at the AV node. The sympathetic nerves are distributed at the SA and AV nodes and all over the heart, especially the ventricles. Sympathetic stimulation increases the SA node rate of discharge, increases cardiac excitability, and increases the force of contraction.1,2
Coronary Circulation
The coronary arteries furnish the heart with its blood supply. The main coronary arteries are on the surface of the heart, but smaller arteries penetrate the heart muscle to provide it with nutrients. The inner surface of the heart derives its nutrition directly from the blood in its chambers.
The coronary arteries originate at two orifices just above the aortic valve. The right coronary artery descends by the right atrium and ventricle and usually terminates as the posterior descending coronary artery. The left coronary artery is usually approximately 1 cm in length and divides into the anterior descending and circumflex arteries. The anterior descending artery usually terminates at the apex of the heart and anastomoses with the posterior descending artery. The anterior descending artery supplies part of the left ventricle, the apex of the heart, and most of the interventricular septum.
The left circumflex artery descends posteriorly and inferiorly down to and terminates in the left marginal artery or, alternatively, may communicate with the posterior descending coronary artery. Venous drainage is with superficial and deep circuits. The superficial veins empty into either the coronary sinus or the anterior cardiac veins, both of which drain into the right atrium. The deep veins drain into the thebesian or sinusoidal channels.
The regulation of coronary blood flow is determined primarily with the oxygen tension of the cardiac tissues. The most powerful vasodilator of the coronary circulation is hypoxemia. Other factors that can affect coronary blood flow are carbon dioxide, lactate, pyruvate, and potassium, all of which are released from the cardiac muscle. Coronary artery steal occurs when collateral perfusion of the myocardium is significantly reduced by an increase in blood flow to a portion of the myocardium that is normally perfused. More specifically, drug-induced vasodilatation of normal coronary arterioles can then divert or steal blood flow from potentially ischemic areas of the myocardium perfused by the vessels that have increased resistance (atherosclerotic vessels). Coronary artery steal can occur when arteriolar-vasodilating drugs such as nitroprusside and isoflurane (Forane) are administered. This situation is especially likely to occur in people who are “stealprone”; they constitute approximately 23% of the patients with coronary artery disease, especially patients who have significant stenosis and occlusions to one or more coronary arteries.1,2
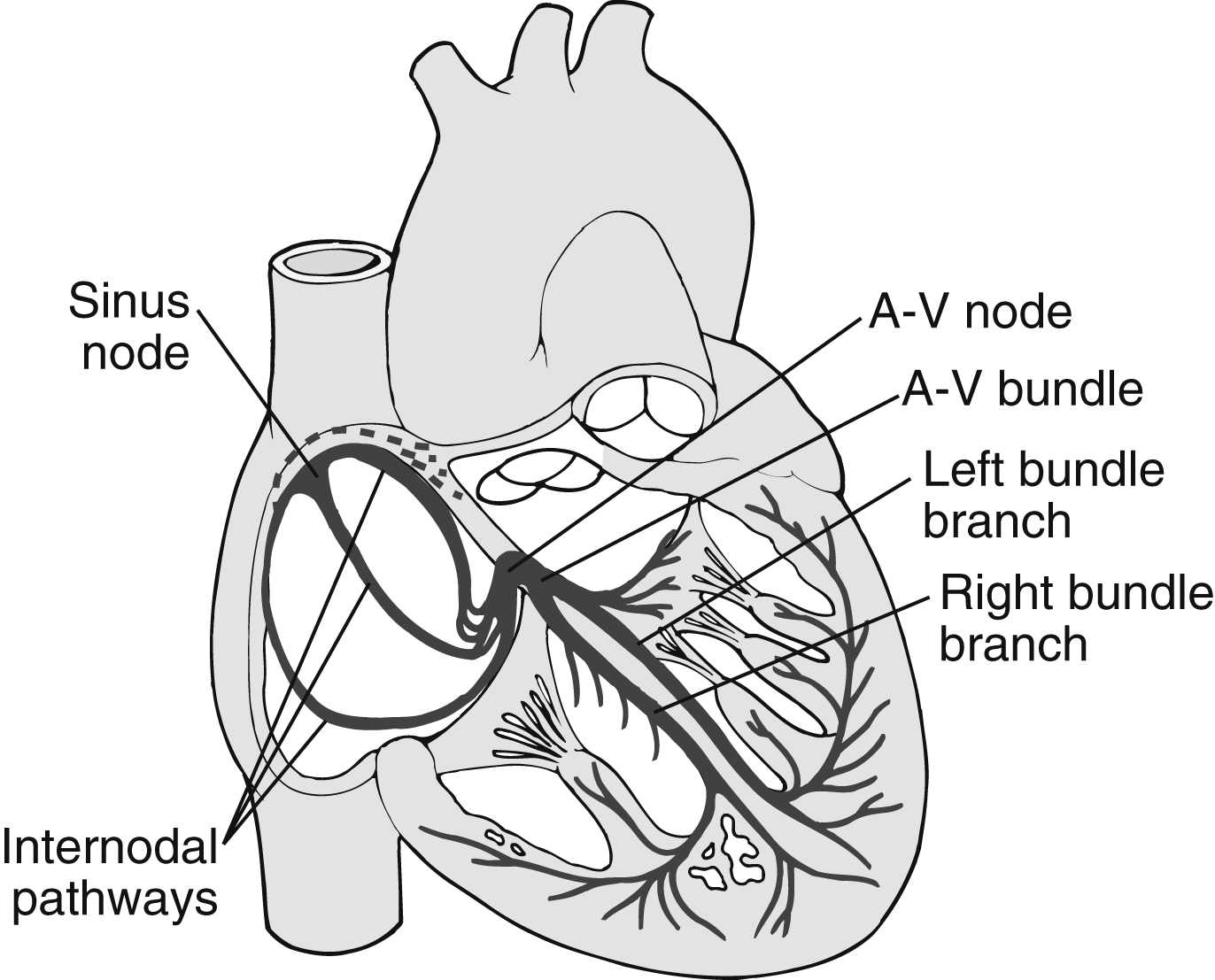
FIG. 11.4 Sinoatrial node and Purkinje’s system of the heart. A-V, Atrioventricular. (From Hall JE: Guyton and Hall textbook of medical physiology, ed 13, Philadelphia, PA, 2016, Elsevier.)
Stimulation of the parasympathetic nervous system causes an indirect decrease in coronary blood flow. Direct stimulation is slight because of the sparse amount of parasympathetic nerve fibers to the coronary arteries. The sympathetic nervous system serves to increase coronary blood flow both directly (as a result of the action of acetylcholine and norepinephrine) and indirectly (caused by a change in the activity level of the heart). The coronary arteries have both alpha and beta receptors in their walls (see the section on adrenergic and cholinergic receptors in Chapter 23).12
Because so much cardiac disease involves the coronary arteries, the anesthetic risk rate increases in patients with cardiac disease. A functional classification of cardiac cases is based on the ability to perform physical activities (Box 11.2). Patients in classes III and IV have a significant risk for surgery and anesthesia and should undergo complete monitoring when they receive care in the PACU.13
Effect of Anesthesia on the Heart
Cardiac dysrhythmias are observed in approximately 60% of all patients who undergo anesthesia.14–15 The inhalation anesthetics, such as isoflurane, sevoflurane, and desflurane, can evoke junctional rhythms or increase ventricular automaticity or both. These anesthetics also slow the rate of SA node discharge and prolong the bundle of His-Purkinje and ventricular conduction times. Along with these changes in rhythm, alterations in the balance of the autonomic nervous system between the parasympathetic and sympathetic systems caused by drugs such as anticholinergics and catecholamines or by light anesthesia can initiate cardiac dysrhythmias. Therefore, in the immediate postoperative period, cardiac dysrhythmias are likely because of light anesthesia during emergence, patient factors such as preexisting comorbidities, and because of the administration of drugs that alter sympathetic activity and fluid shifts intraoperatively, which can cause electrolyte imbalances. Consequently, continuous monitoring of cardiac rate and rhythm is mandated in the PACU.14,15 With the increased use of regional anesthesia for procedures in the perioperative setting, signs and symptoms of anesthesia toxicity must be monitored. Regional blocks using epinephrine in conjunction with sedation medications may have an adverse effect on the cardiovascular system. Symptoms initially present as hypertension and tachycardia, which untreated can lead to progressive hypertension and dysrhythmias. Dependent on the medications administered, side effects involving the cardiovascular system can by masked by sedation medication.16
Myocardial Infarction
Acute myocardial infarction is a commonly encountered medical emergency that can occur in the PACU. More than 90% of myocardial infarctions result from disruption of an atherosclerotic plaque with subsequent platelet aggregation and formation of an intracoronary thrombus. The form of myocardial infarction that results depends on the location and the degree of coronary obstruction as well as associated ischemia. A partially occlusive thrombus is the typical cause of non–ST elevation myocardial infarction. At the other end of the spectrum, if the thrombus completely obstructs the coronary artery, the results are more severe ischemia and a larger amount of necrosis, manifesting as an ST-elevation myocardial infarction.15,17 The objectives in the management of a patient with an acute myocardial infarction are pain relief, control of complications, salvage of ischemic myocardium, and a return to a productive life. The diagnosis of myocardial infarction is made based on clinical findings, and therapy should be instituted immediately when suspected. (Cardiopulmonary resuscitation is discussed in Chapter 57.) An electrocardiogram performed in the PACU may reveal an injury pattern, but normal electrocardiographic results certainly do not exclude a diagnosis of myocardial infarction.17
Physical assessment for a suspected myocardial infarction can include the following subjective findings: (1) pain or pressure, which is usually substernal but may be manifested in the neck, shoulder, jaws, arms, or other areas; (2) nausea; (3) vomiting; (4) diaphoresis; (5) dyspnea; and (6) syncope. The onset of pain can occur with activity but can also occur at rest. The duration may be prolonged, from 30 minutes to several hours. Objective findings can include hypotension, pallor, and anxiety. The blood pressure, pulse, and heart sounds may be normal with an acute myocardial infarction. On auscultation of the chest, the abnormal cardiac findings may include atrial gallop, ventricular gallop, paradoxical second heart sound, friction rub, and abnormal precordial pulsations.17
The electrocardiographic pattern can vary by the location and extent of the infarction, but myocardial damage can occur without changes in the electrocardiogram. Some typical features of a transmural infarction are acute ST-segment elevation in leads that reflects the area of injury, abnormal Q waves, and T wave inversion.
Serum Markers of Infarction
Necrosis of myocardial tissue causes disruption of sarcolemma so the intracellular macromolecules leak into the bloodstream. Detection of such molecules in the serum, particularly cardiac-specific troponin and creatine kinase MB (CK-MB) isoenzyme, serves important diagnostic and prognostic roles.8–11
Troponin is a cardiac-specific regulatory protein in muscle cells that controls interactions between myosin and actin. Although found in both skeletal and cardiac muscles, the cardiac forms of troponin I and troponin T are structurally unique, and highly specific assays for their detection in the serum have been developed. Cardiac troponin serum levels begin to rise 3 to 4 hours after the onset of chest discomfort, peak between 18 and 36 hours, then decline slowly, allowing for detection for up to 10 to 14 days after a large infarction.8–11
In the absence of trauma, the elevation of CK-MB is highly suggestive of myocardial injury. To facilitate the diagnosis of infarction using this marker, it is common to calculate the ratio of CK-MB to total CK. The ratio is usually greater than 2.5% in the setting of myocardial injury and less than that when CK-MB is from another source. The serum level of CK-MB starts to rise 3 to 8 hours after infarction, peaks at 24 hours, and returns to normal within 48 to 72 hours.8–11
Research studies have shown that patients who have had a myocardial infarction within 6 months before surgery have a recurrence rate of 54.5% for a myocardial infarction that could occur during or after the surgical procedure. If the myocardial infarction occurred between 6 months and 2 years before surgery, the rate of recurrence of infarction is between 20% and 25%. Between the second and third years, the incidence rate of reinfarction is approximately 5%. Most studies indicate that 3 years after the original myocardial infarction, the recurrence rate is approximately 1%, which equals the normal rate of myocardial infarction in the general population. Therefore, the chance of a patient having an acute myocardial infarction in the PACU can be considered significant. This chance is especially true for patients in the PACU who have had a myocardial infarction within the past 3 years, who have a documented myocardial infarction risk factor (e.g., angina, hypertension, diabetes), or who have some combination of the previous factors.17,18
Perianesthesia Nursing Care
The perianesthesia nurse should be constantly alert for complications such as anxiety, arrhythmias, shock, left ventricular failure, and pulmonary and systemic embolisms. Pain and apprehension can be relieved with treatment with morphine sulfate, fentanyl, or hydromorphone (Dilaudid). Oxygen should be administered with nasal prongs because a face mask may increase the patient’s apprehension. Continuous cardiac monitoring should be instituted, and the patient should be kept in a quiet area. Drugs such as atropine, lidocaine, digitalis, quinidine, sodium nitroprusside (SNP), phentolamine, and nitroglycerin should be available. A machine for countershock also should be immediately available. Fluid therapy and urine output should be monitored completely for prevention of fluid overload. A pulmonary artery catheter or central venous pressure (CVP) monitor may be used for determining fluid replacement in patients with reduced intravascular volume and hypotension (see the discussion of CVP catheters in the following section). A benign myocardial infarction does not exist; all patients with a diagnosed myocardial infarction need constant competent perianesthesia care.9,19
Central Venous Pressure Monitor
The CVP monitor enhances the assessment of venous return and hypovolemia. More specifically, the CVP monitor is used to assess the adequacy of central venous return, blood volume, and right ventricular function. The actual pressure reading obtained from this monitor reflects the pressure in the great veins when blood returns to the heart.
The left ventricular end-diastolic pressure (LVEDP) serves as a good indicator of left ventricular preload. In a patient with a good ejection fraction (greater than 65%), the CVP measurement serves as an approximate value for the LVEDP. However, it is important to remember that the CVP has limited value in assessment of left ventricular hemodynamics.
In the immediate postoperative setting, the CVP remains an excellent parameter indicating the adequacy of blood volume. In the hypovolemic state, the CVP is decreased. The administration of appropriate fluids and blood to expand the intravascular space increases the CVP toward the patient’s baseline reading. In the clinical setting, no absolute predetermined normal value for a CVP reading exists. The best use of this particular monitoring mode is for serial measurements for assessment of cardiovascular performance. See Chapter 27 for a discussion of the CVP monitor.4,11,13
Pulmonary Artery Catheter
The pulmonary artery catheter is used to monitor the central venous, pulmonary artery, and pulmonary capillary wedge pressures. This balloon-tipped catheter with four or five ports is discussed in detail in Chapter 27. Pulmonary artery catheters are used predominantly for cardiothoracic surgical patients and surgical patients with large volume shifts.
In the immediate postoperative period, the pulmonary artery catheter is usually used for patients with clinical shock, compromised ventricular function, and severe cardiac or pulmonary disease. In addition, patients who have had extensive surgical procedures or major cardiovascular surgery can benefit from this monitor. Accurate monitoring of left-sided and right-sided preload along with the rapid determination of cardiac output makes this monitor an excellent parameter for determining mechanical and pharmacologic therapy with the intended outcome of enhanced cardiac performance and tissue perfusion.11,13
Circulatory System
Red Blood Cells
The healthy red blood cell (RBC) is in the form of a biconcave disk that can change its shape to move through the microcirculation. The major function of the RBC is the transport of oxygen to the tissue cells; it is also an important factor in carbon dioxide transport. The RBC is responsible for approximately 70% of the buffering power of whole blood in the maintenance of acid-base balance.
RBCs are produced by the bone marrow. The normal rate of production is sufficient to form approximately 1250 mL of new blood per month. This rate is also the normal rate of destruction. The average life span of an RBC is 120 days. The hematocrit value is the percentage of RBCs in the blood. The optimal hematocrit range in adults is between 30% and 42%. When the hematocrit level is reduced to less than 30%, the oxygen-carrying capacity declines steeply. Moreover, when the hematocrit level rises to greater than 55%, the oxygen-carrying capacity declines because the increase in blood viscosity causes increased work for the heart and decreased cardiac output. The normal amount of hemoglobin in the RBC ranges from 10 to 13.5 g. In fact, the amount and type of hemoglobin determine the oxygen-carrying capacity. Recent evidence indicates that the cutoff value for risk of reduced oxygen-carrying capacity and blood volume is a hemoglobin level of 11 g, a hematocrit of 27%, or both. Transfusion with blood or blood products to raise the level of hemoglobin should be strongly considered for any patient with values lower than the cutoff values.2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree