1. The septum transversum forms in the neck from the third, fourth, and fifth cervical myotomes and “descends” with the heart into the developing pleural cavity, subsequently separating into three layers.
2. Between the eighth and tenth weeks of fetal life, the diaphragm is derived from the septum transversum, the two pleuroperitoneal membranes, and the dorsal mesentery of the esophagus.
3. The mesenchyme of the septum transversum originally forms in the neck from the third, fourth, and fifth cervical myotomes, explaining the innervation of the central tendon of the diaphragm as well as the etiology of referred shoulder pain with diaphragmatic stimulation.
4. Because of the abbreviated length of the ventral mesentery and the communication between the extraembryonic coelom and the peritoneal cavity, rapidly growing peritoneal cavity contents herniate and extraembryonic looping of abdominal contents can take place.
5. Because the diaphragmatic malformation originates early in embryogenesis (normal closure by the seventh week), the lung parenchyma and the pulmonary circulation are usually hypoplastic, and the heart and mediastinum are often displaced to the right (diaphragmatic hernias (DHs) are five times more common on the left than the right).
6. Respiratory failure due to congenital DH may be caused by lung parenchyma and pulmonary vascular hypoplasia, pneumothorax due to decreased compliance, and mediastinal shifting.
7. Extracorporeal membrane oxygenation (ECMO) may be required in the perioperative period because of pulmonary vascular reactivity and respiratory distress refractory to conventional high-frequency or oscillatory ventilation.
8. Following a Nuss repair of a pectus excavatum, agitation and pain should be minimized from the time of anesthetic emergence and for the first 3 to 4 days postoperatively so that the risk of displacing the bar is minimal.
9. A range of abdominal wall defects may occur as a result of residual patency of the umbilicus and the omphalomesenteric duct, the most urgent of which are omphaloceles, sometimes confused in the neonatal period with gastroschisis. Both defects in the abdominal wall of the neonate result in herniation of abdominal contents to varying degrees and are urgent neonatal surgical emergencies because of the threats of ischemia, bowel obstruction, and sepsis.
I. Development of the body cavity and wall
A. The major body cavities—the thoracic or pleural, the abdominal or peritoneal, and the pericardial—are the result of the partitioning of the intraembryonic coelom, which begins its development in the third week of embryogenesis, following the formation of initial germ layers, the endoderm and ectoderm, on the 12th day.
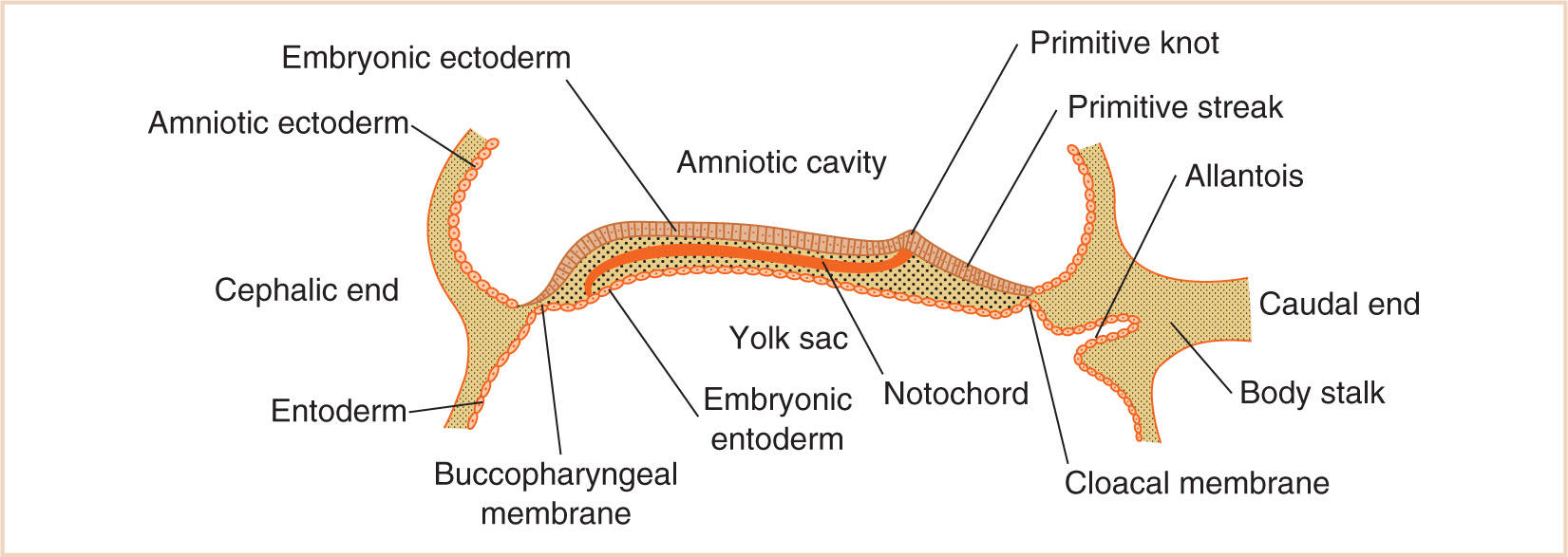
B. Although the two germ layers form the bilaminar embryonic disc (upper surface of embryonic ectoderm and the lower surface of the embryonic endoderm) (Fig. 17.1), the development of the third germ layer, the embryonic mesoderm, is delayed until the ectodermal cells of the primitive knot (Hensen node) invest a space between the ectoderm and endoderm by extending laterally, anteriorly, and posteriorly on either side of the notochord.
C. Intriguingly, the mesoderm does not extend to the most cephalic end of the embryonic disc, where ectodermal and endodermal layers fuse over the small area that eventually becomes the buccopharyngeal membrane, or the most caudal end, where the ectodermal and endodermal layers fuse to become the cloacal membrane.
D. Embryonic mesoderm differentiates into paraxial mesoderm (Fig. 17.2), which begins to segment into approximately 43 pairs of somites about the fourth week of development.
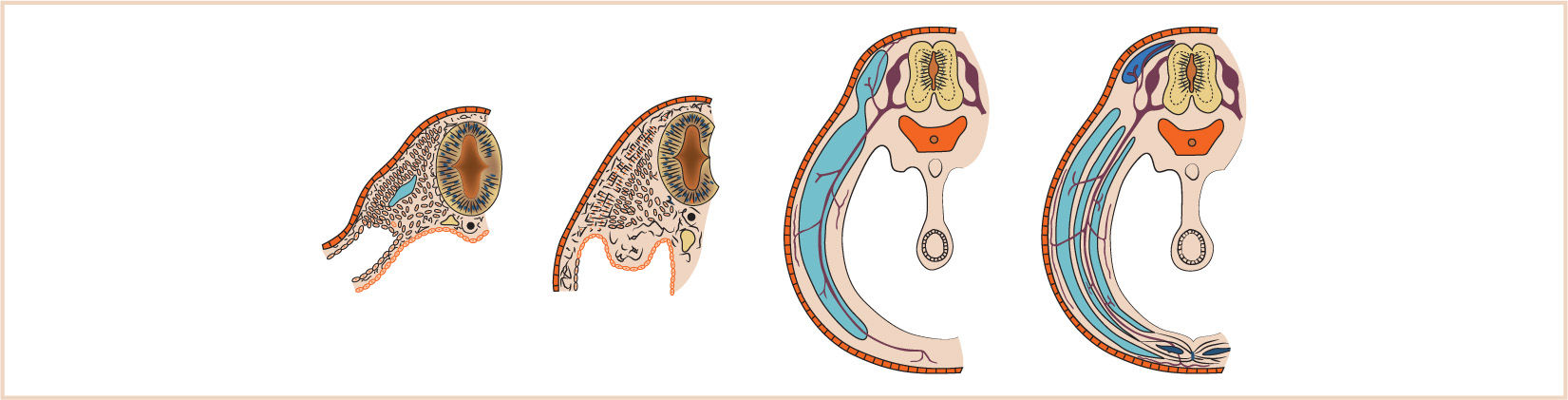
FIGURE 17.2 Development of the paraxial mesoderm and subsequent differentiation into the ventromedial sclerotome and dorsolateral dermomyotome.
E. The somites further differentiate into a ventromedial (sclerotome) and dorsolateral portion (dermomyotome), with further differentiation into a myotome (from which segmentally based skeletal muscle derives) and a dermatome (from which dermis and subcutaneous tissue derives, innervated by that somite’s nerve supply).
F. The lateral mesoderm splits into a somatic and splanchnic layer and encloses the body cavity known as the intraembryonic coelom, which eventually forms the pleural and peritoneal cavities.
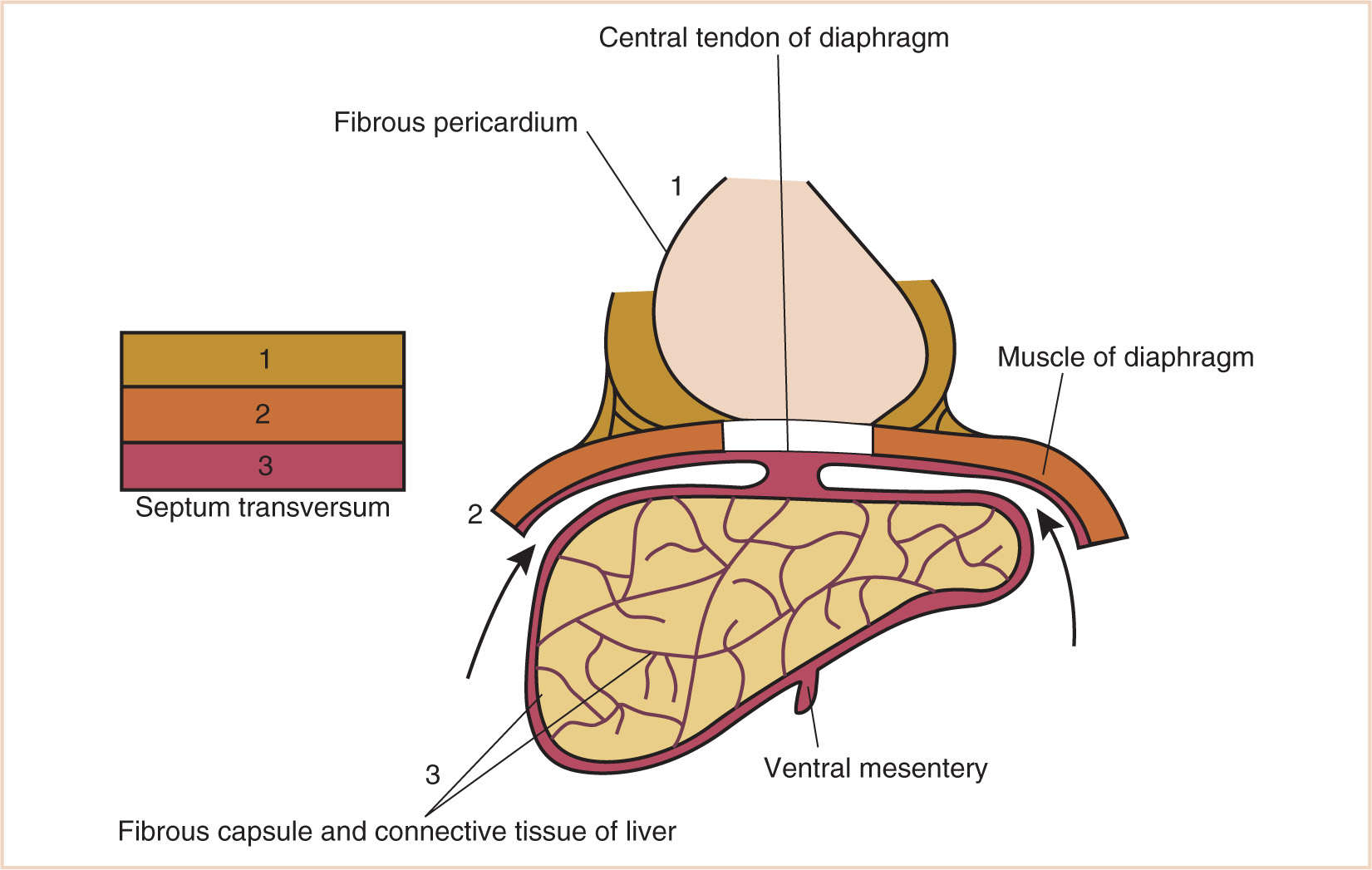
G. The septum transversum forms in the neck from the third, fourth, and fifth cervical myotomes and “descends” with the heart into the developing pleural cavity, subsequently separating into three layers (Fig. 17.3).
H. The cranial-most layer forms the fibrous pericardium; the middle layer forms the muscles of the diaphragm, the central tendon of the diaphragm, and central areas of the pleural and peritoneal covering of the diaphragm; and the caudal-most layer forms the fibrous capsule and connective tissue of the liver and the ventral mesentery of the developing midgut.
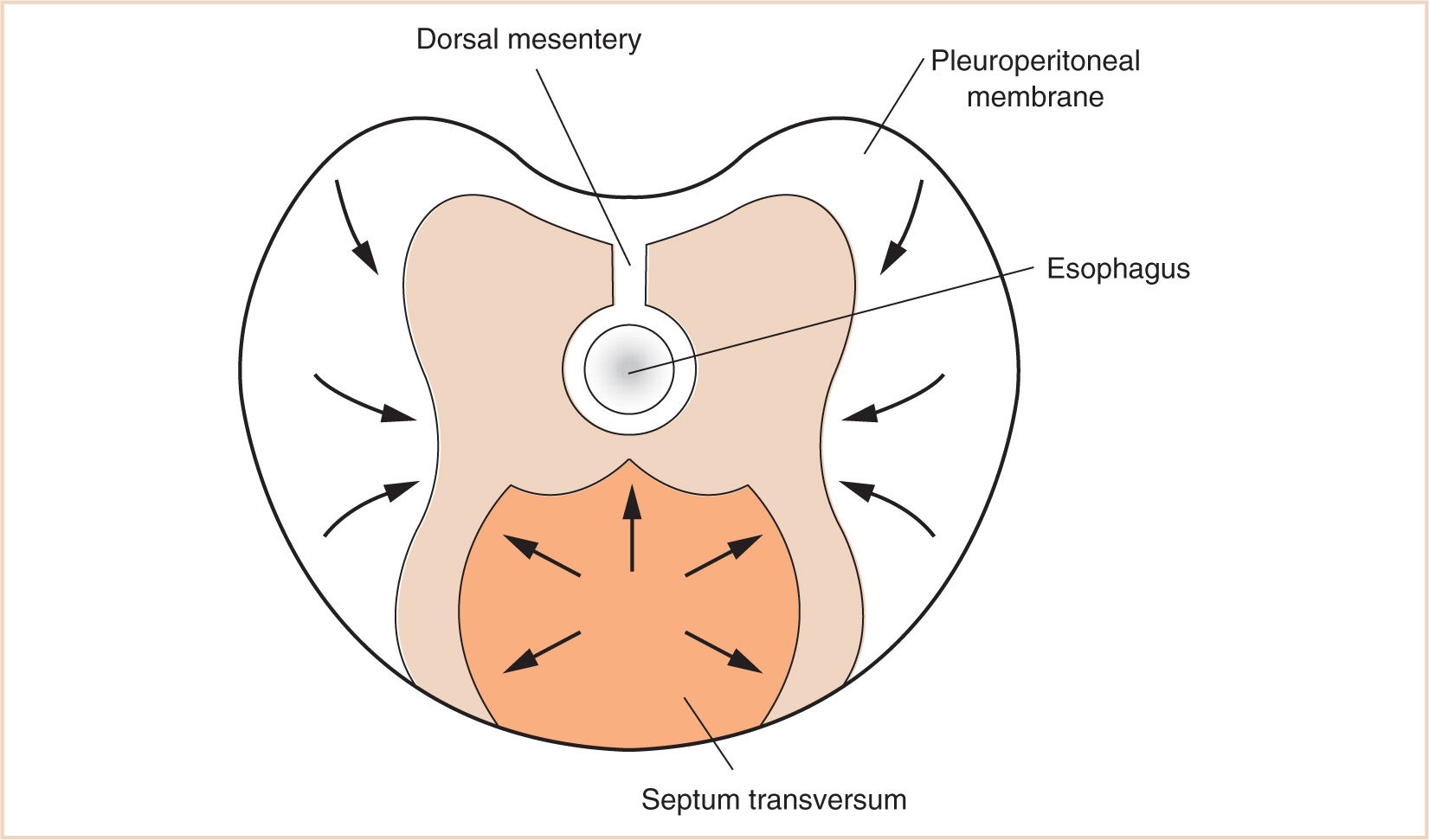
I. Between the eighth and tenth weeks of fetal life, the diaphragm is derived from the septum transversum, the two pleuroperitoneal membranes, and the dorsal mesentery of the esophagus.
J. The mesenchyme of the septum transversum originally forms in the neck from the third, fourth, and fifth cervical myotomes, explaining the innervation of the central tendon of the diaphragm as well as the etiology of referred shoulder pain with diaphragmatic stimulation (Fig. 17.4).
K. The fate of the pleuroperitoneal membranes on either side is to continue to grow medially until fusing with the septum transversum and close the pleuroperitoneal canal.
1. This tissue, derived from body somites, remains innervated by T6-12. Failure of fusion of the different elements that form the diaphragm results in a DH through persistence of the pleuroperitoneal canal, a defect in the space between the sternal and costal margins of the diaphragm or an abnormal patency of the esophageal hiatus, resulting in a hiatal hernia.
2. Eventrations of the diaphragm may also occur as a result of discrete dysplastic areas of diaphragmatic muscle.
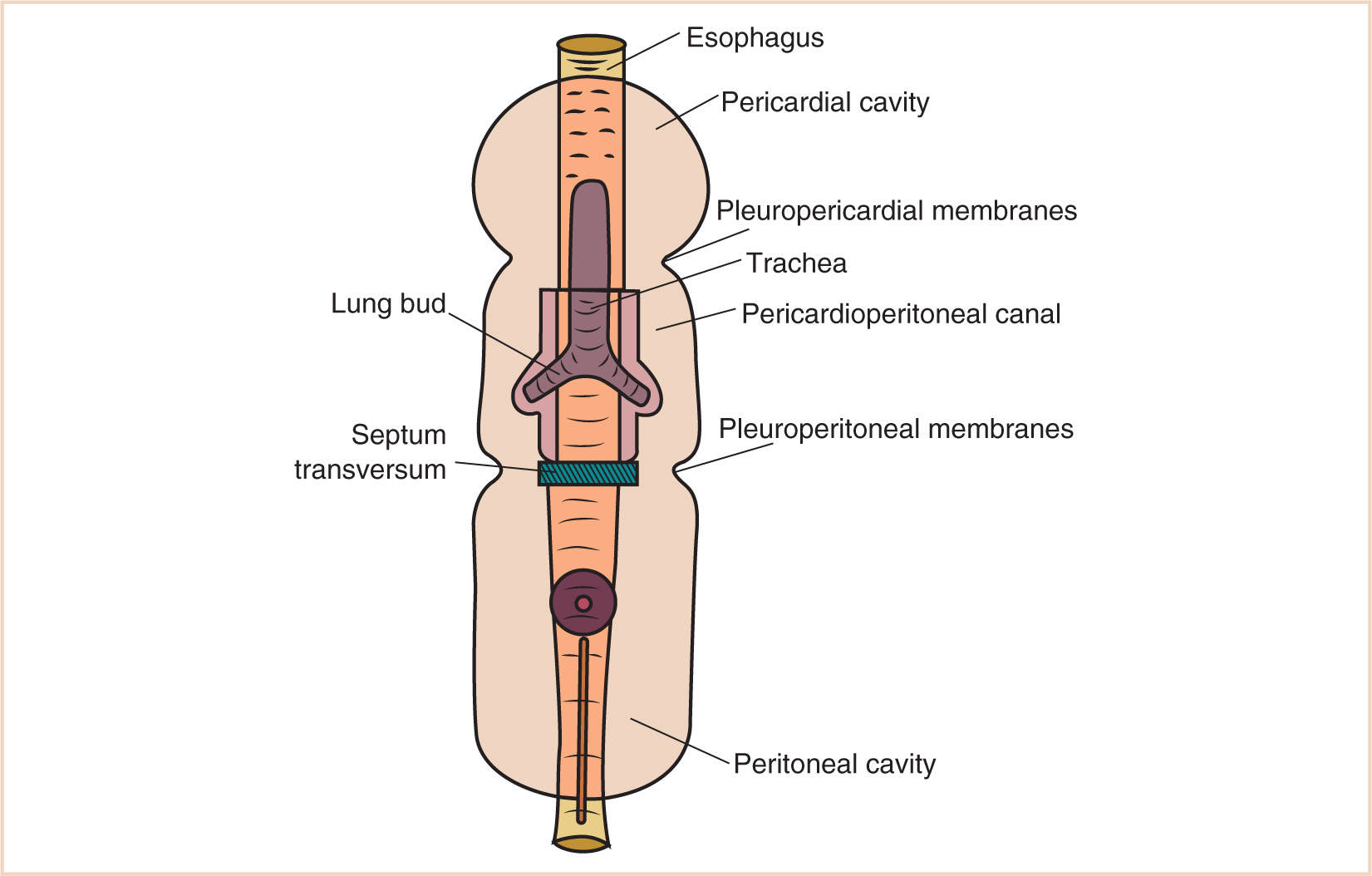
L. The most cranial portion of the intraembryonic coelom is the pericardial cavity.
1. Its initial formation is cranial to the buccopharyngeal membrane, but with the development of the head fold, the pericardium and the endocardial heart tube rotate so that the pericardial cavity lies ventral to the pharynx.
2. The pericardial cavity in this position and at this time communicates freely with the peritoneal cavity through the pericardioperitoneal canals (Fig. 17.5).
3. As the lung buds branch from the tracheal diverticulum that arises out of the primitive foregut, they invaginate the surrounding splanchnopleuric mesoderm that covers them and further invest the surrounding pericardioperitoneal canals.
M. The canals become the pleural cavity on each side, which now enlarge to accommodate the developing heart.
1. As the embryo elongates, more so in a craniocaudal manner, the pericardial cavity descends from the pharyngeal area to the thorax.
2. The surrounding pleuropericardial canal initially remains continuous with the pleuroperitoneal canal but then is divided by the formation of the pleuropericardial membranes, which will eventually fuse with the septum transversum and the dorsal mesentery to form the diaphragm. Therefore, the pleural and peritoneal cavities become separated.
N. The peritoneal cavity is formed from the remaining intraembryonic coelom caudal to the septum transversum, the developing diaphragm.
1. Originally freely communicating with the extraembryonic coelom, the peritoneal cavity will eventually communicate only with the extraembryonic coelom through the umbilical cord.
2. Moreover, the developing dorsal and ventral mesenteries will further divide the cranial-most portion of the peritoneal cavity into right and left halves, up to the level of the umbilicus.
3. This most cranial portion is crucial, however, to the formation of the greater and lesser peritoneal sacs, with the dorsal mesentery forming the mesenteries of the small and large intestines and the greater omentum and the ventral mesentery, the falciform ligament and the ligamentum teres and the lesser omentum.
4. Moreover, because of the abbreviated length of the ventral mesentery and the communication between the extraembryonic coelom and the peritoneal cavity, rapidly growing peritoneal cavity contents herniate and extraembryonic looping of abdominal contents can take place.
O. The umbilicus is the portion of the anterior abdominal wall attached to the umbilical cord. Relegated to an ignominious status after birth, during fetal life it is the most essential housing structure for growth and development of the fetus, containing the right and left umbilical arteries, the umbilical vein, the vitelline duct, the allantois, and the extraembryonic coelom.
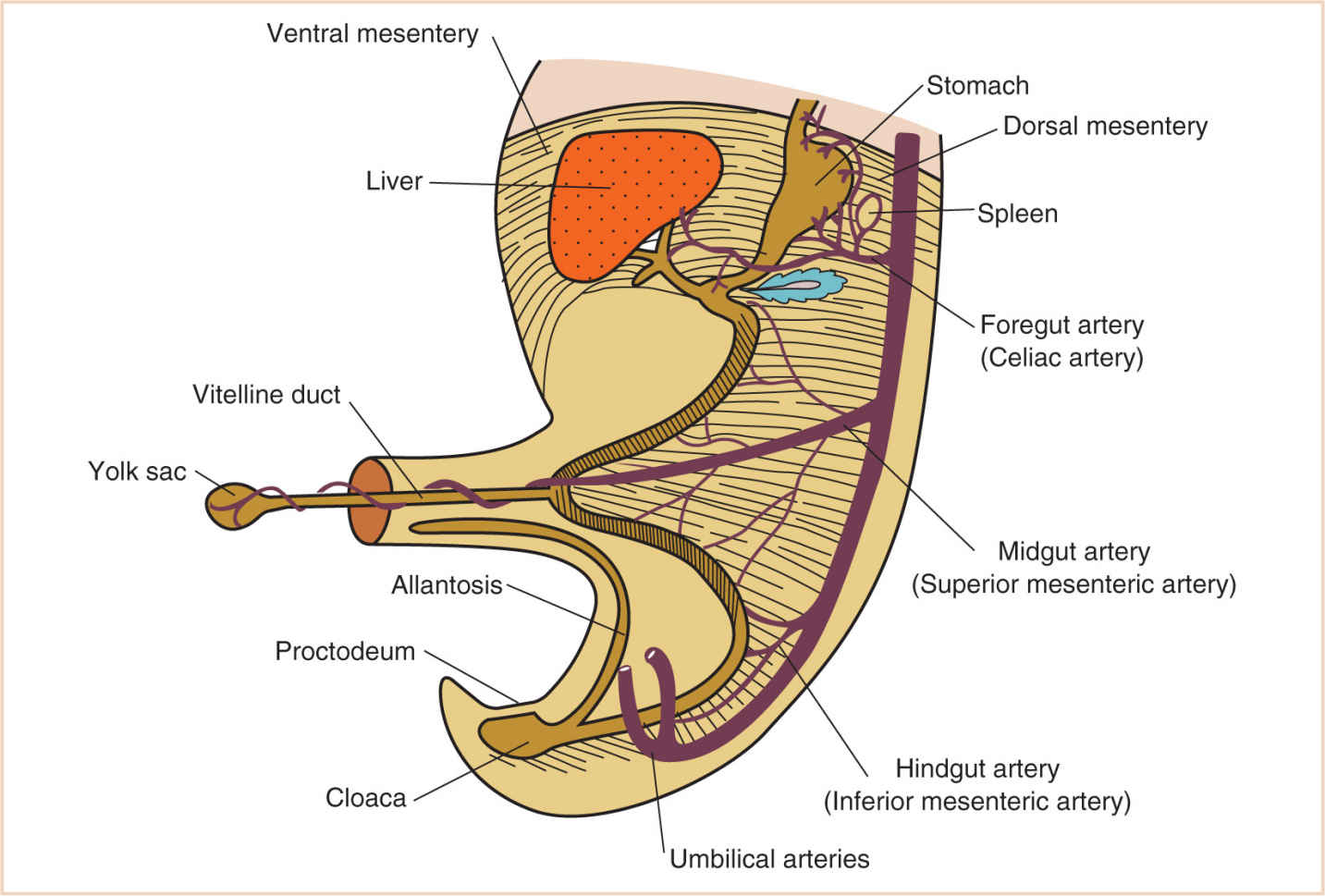
FIGURE 17.6 Developing intra-abdominal structures in the region of the vitelline duct and the eventual umbilicus.
1. In addition to these vital structures, it is the space that permits the extrusion of abdominal contents in the growing embryo from the 6th to the 12th week of development.
a. Failure of any of these structures to develop and regress normally can result in growth restriction where there should not be any or persistent spaces where none should exist, such as umbilical hernia, patent omphalomesenteric duct, and omphalocele.
P. A defect in the anterior abdominal wall adjacent to the umbilicus will result in a gastroschisis, with normal attachment of the umbilical cord to the abdominal wall (Fig. 17.6).
II. Abnormalities of the body cavity and wall
DISORDER: The diaphragm: congenital DH, diaphragmatic eventration
A. Background
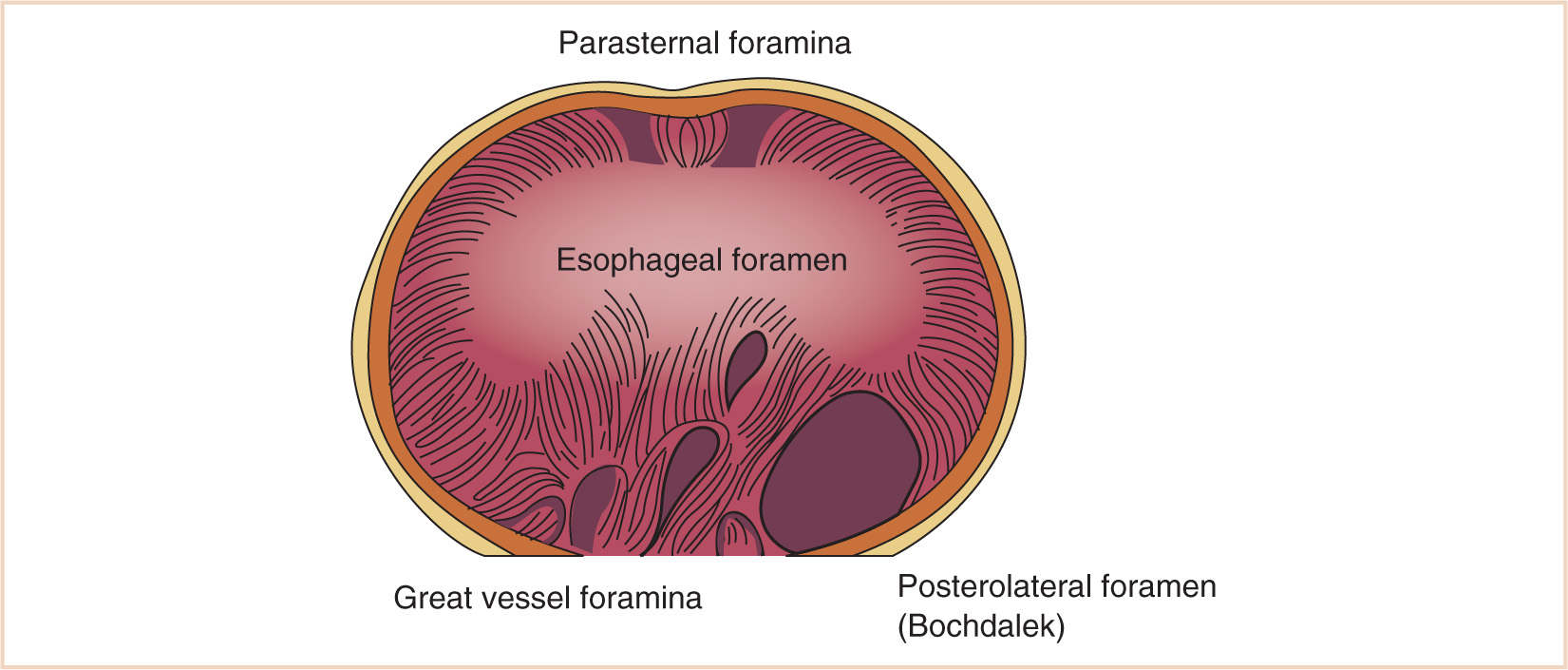
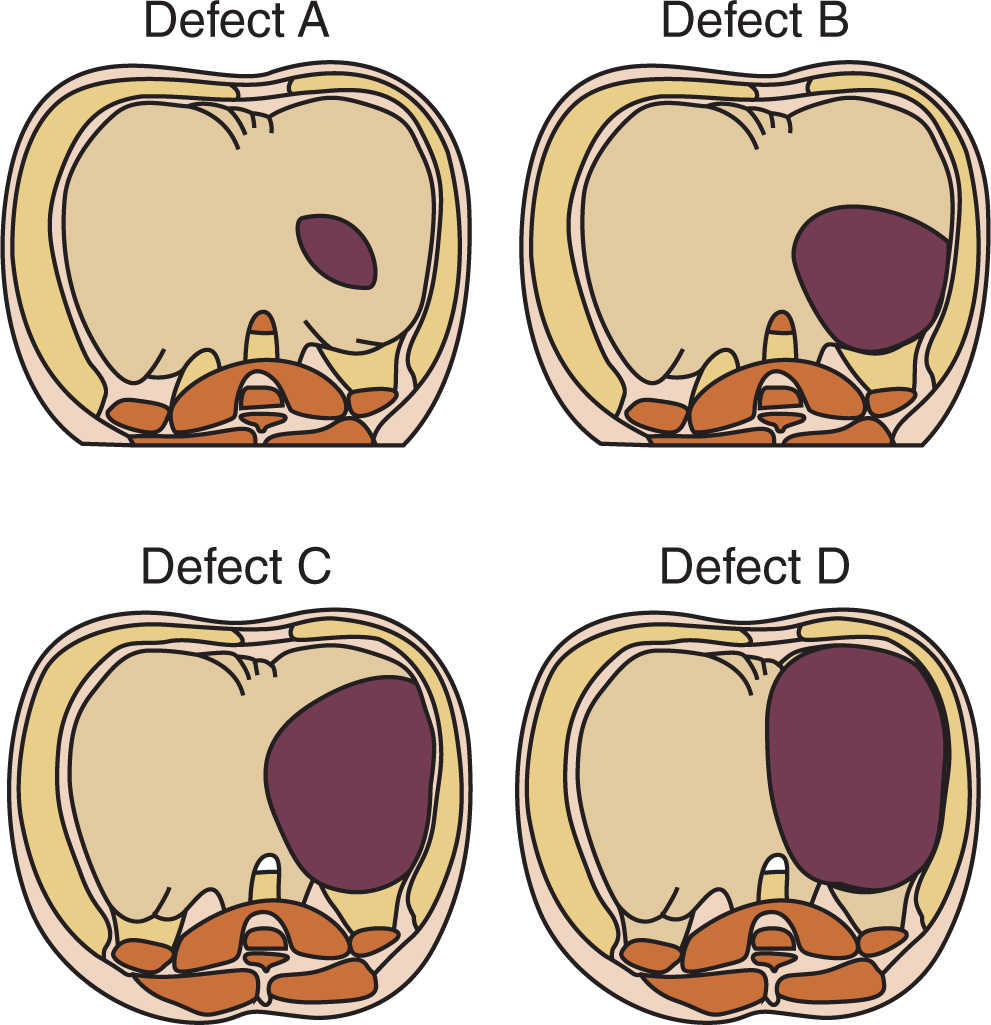
1. Failure of fusion of the components of the diaphragm (septum transversum, pleuroperitoneal membrane) may result in paraesophageal, retrosternal, or, the most common form, persistent pleuroperitoneal canal (posterolateral) herniation of abdominal contents into the chest.
a. Because the diaphragmatic malformation originates early in embryogenesis (normal closure by the seventh week), the lung parenchyma and the pulmonary circulation are usually hypoplastic, and the heart and mediastinum are often displaced to the right (DHs are five times more common on the left than the right).
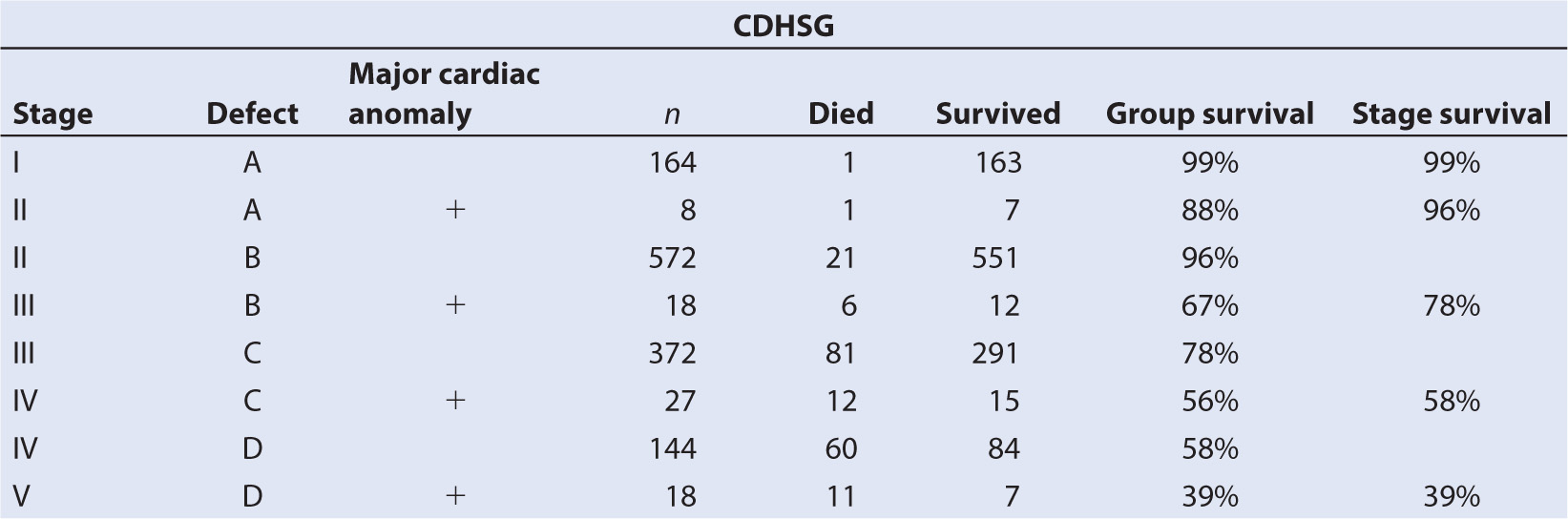
b. The Congenital DH Study Group (CDHSG) has proposed a standardized reporting system for congenital DH and has related this taxonomy to survival (1) (Table 17.1).
c. Moreover, the size of the defect is directly correlated with mortality rate, prevalence of other anomalies, particularly cardiovascular malformations, and number of abnormal systems. It is also correlated inversely with gestational age, body weight, and the prevalence of a hernia sac (2).
B. Embryology/anatomy
1. Hernia: Failure of fusion of peripheral and central elements of the diaphragm, occurring in newborns.
a. The left posterolateral foramen occurs most often and is the typical newborn presentation.
b. Hernias in other sites may present later in life than the newborn period (Fig. 17.7).
2. Eventration: Impaired development of the central tendon of the diaphragm or failure of phrenic nerve innervation of a hemidiaphragm with cranial repositioning of the paralyzed hemidiaphragm.
C. Physiologic considerations
1. Respiratory distress due to lung parenchyma and pulmonary vascular hypoplasia; there may be associated pneumothorax due to decreased compliance and mediastinal shifting.
2. Hypoxemia, hypercarbia, acidemia, metabolic acidosis
3. Pulmonary hypertension
4. Right-to-left shunting due to increased pulmonary vascular resistance; may be due to patency of the ductus arteriosus or at the atrial level
5. Malrotation may occur in >50% and up to 100% of patients
6. Newborn considerations: hypoglycemia, hypocalcemia, contraction alkalosis due to nasogastric (NG) suction for bowel obstruction
D. Surgical repair
1. Typically supine position with transabdominal subcostal incision. Intrathoracic approach is usually through right posterior thoracotomy for right-sided defects.
2. By using laparotomy, herniated viscera is removed from chest cavity and relocated to abdomen.
3. Primary repair of diaphragm if there is adequate mobilization; otherwise, prosthetic (Silastic or Gortex) material is used.
4. Visceral adhesions may need to be lysed or the Ladd procedure carried out to fixate mobile viscera postreduction of the malrotation.
5. Primary closure of abdomen if tension is minimal; otherwise, Silastic pouch closure.
E. Anesthesia issues
1. Readiness for surgery
a. Preoperative respiratory distress will often result in intubation/mechanical ventilation before the surgical procedure. In some cases, patients will have been or will currently be receiving ECMO.
b. Preoperative laboratory tests include arterial blood gas analysis, complete blood count (CBC), electrolytes, glucose, blood urea nitrogen (BUN), creatinine, chest x-ray (CXR), echocardiogram (ECG), and coagulation studies.
c. Low peak inspiratory pressures should be maintained for transport and in the initial phase of ventilation in the operating room.
2. Anesthesia goals
a. Adequate gas exchange
b. Monitoring and replacement of electrolytes; attention to metabolic alkalosis because of preoperative NG suction; attention to metabolic acidosis because of impaired perfusion, mediastinal shifting, and visceral herniation; attention to respiratory acidosis because of respiratory distress and pulmonary hypoplasia
c. Prevention of aspiration
d. Perioperative pain control
3. General anesthesia
a. Position: Supine
b. Typical surgical time: 2.0 to 2.5 hour
c. Induction
(1) Full stomach precautions; aspiration of gastric contents with NG tube before induction.
(2) If the infant is not already intubated, an awake intubation with direct laryngoscopy or a rapid sequence intravenous (IV) induction of anesthesia should be planned. Preoxygenation and cricoid pressure are required; then, a rapid sequence administration of sodium thiopental or propofol should be carried out.
(3) Muscle relaxation using succinylcholine with preceding or concomitantly administered atropine is typically utilized. Many now choose to administer rocuronium or mivacurium in doses designed to provide laryngeal relaxation within 60 to 90 seconds; however, significant oxygen desaturation may occur in these stressed neonates.
(4) Difficult airways should probably be approached with an awake intubation.
d. Equipment: Low compression volume anesthesia breathing circuit (circle absorption system vs. Mapleson D vs. Bain circuit)
e. Monitoring: Standard noninvasive monitoring; pre- and postductal arterial lines can be considered, or preductal pulse oximeter with a postductal arterial line.
f. Maintenance
(1) Opioid and/or inhalation agent technique; combination usually works best. Avoidance of nitrous oxide. Optimal FIO2 to achieve a preductal SpO2 of 95% to 100%.
(2) Neuromuscular blockade with nondepolarizing agent.
(3) Volume support with judicious amounts of crystalloid or colloid. Blood loss is minimal so unlikely to require transfusion.
CLINICAL PEARL Care should be taken to provide a background infusion of 10% dextrose in small infants because of minimal glycogen stores in the liver and muscle in prematures and exprematures.
(4) Monitor for contralateral (usually right-sided) pneumothorax.
(5) Maintain peak inspiratory pressure <30 cm H2O because of pulmonary hypoplasia.
(6) Blood gas monitoring intraoperatively for acidosis, hyponatremia, hypoxemia, hypercarbia, hematocrit, and coagulation.
g. Emergence
(1) Unless DH is mild and patient’s preoperative condition was benign, the infant should likely remain intubated and transported to neonatal intensive care unit (NICU) with full monitoring, respiratory and resuscitation equipment, and drugs available.
h. Perioperative
(1) ECMO may be required in the perioperative period because of pulmonary vascular reactivity and respiratory distress refractory to conventional or even high-frequency or oscillatory ventilation.
(2) Monitoring for hypoglycemia as a result of depletion of marginal endogenous glycogen stores, particularly if infants appear to exhibit signs and symptoms suggestive of hypoglycemia such as jitteriness, cardiovascular instability, or tremors. Ongoing monitoring of metabolic and hematologic status.
(3) Pain management with continuous fentanyl infusion or epidural analgesia.
i. Regional anesthesia
(1) An epidural placed transcaudally or through lumbar or thoracic sites may be chosen to provide abdominal, chest wall, and pleural analgesia around the incisional site.
TABLE 17.2 Congenital chest wall deformities
Pectus excavatum
Pectus carinatum
Cleft sternum
Pentalogy of Cantrell
Asphyxiating thoracic dystrophy (Jeune syndrome)
Spondylothoracic dysplasia (Jarcho–Levin syndrome)
DISORDER: The chest wall: pectus excavatum or carinatum
A. Background
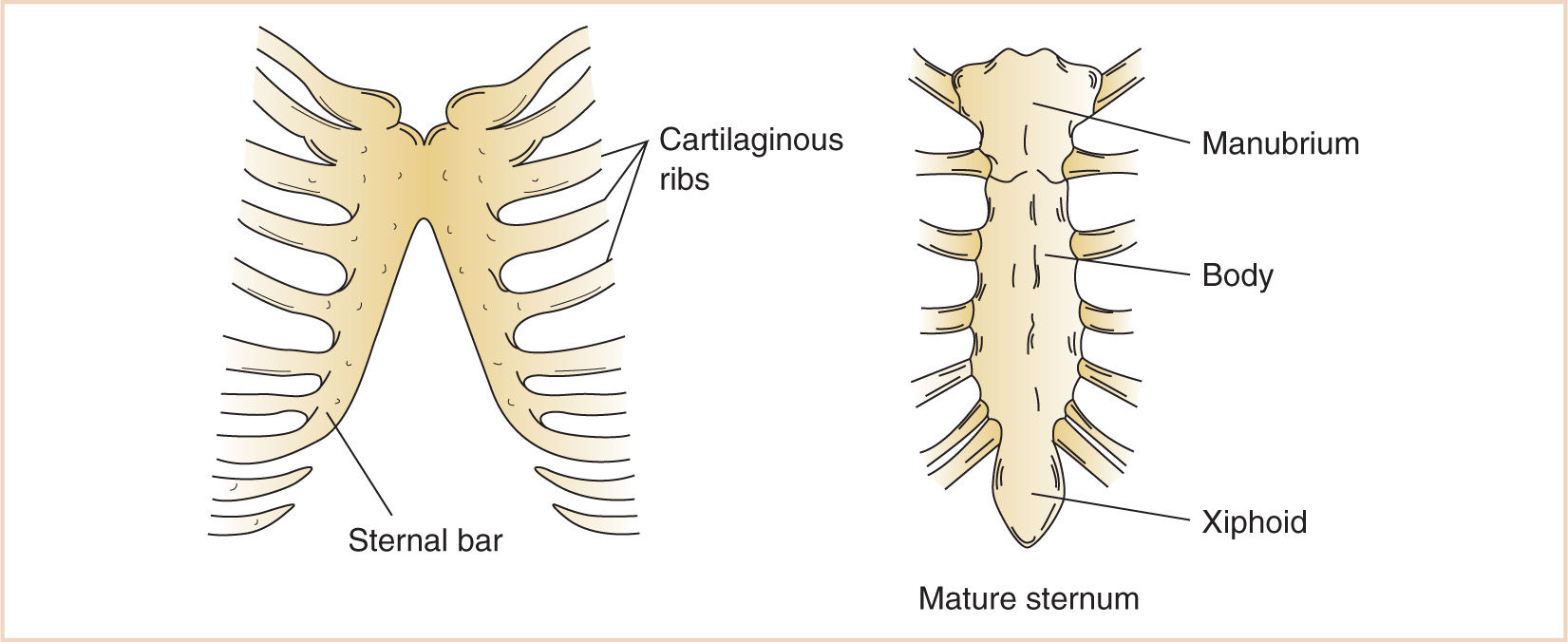
1. Mesenchyme migrating from the body wall chondrifies and fuses in a craniocaudal direction with the rib cartilages that develop from the vertebral bodies, forming the three divisions of the sternum—the manubrium, the body of the sternum, and the xiphoid process.
2. Abnormalities of development or fusion may result in hypoplastic, dysplastic or abnormally fused ribs, or dysplasia of the sternum (Table 17.2).
3. Over the last two decades, there has been a trend toward operating at a later time in adolescence, from 13.5 years of age 20 years ago to about 15.5 years of age currently (3).
4. Bracing may be an alternative to surgical correction of pectus carinatum if patient compliance is good and bracing is begun prior to skeletal maturity (4).
B. Embryology/anatomy
1. The exact developmental abnormality in pectus excavatum or carinatum is unclear, although it is easy to recognize the bony and cartilaginous abnormalities of convexity or concavity (Fig. 17.8).
2. While the genetics remain unclear, familial occurrence in 35% is recognized and is associated with Marfan and Poland syndromes.
3. Usually noted within the first year of life, pectus deformities typically worsen during the rapid bone growth of early adolescence.
C. Physiologic considerations
1. Most patients do not have cardiorespiratory system symptoms even in the presence of mildly abnormal pulmonary function tests (5–9).
2. Mitral valve prolapse has been reported in 20% to 60% of patients. There is some evidence to suggest that improvement in cardiac performance can occur in cardiac-compromised patients postsurgical correction of the pectus deformity.
3. Some patients experience chest and/or back pain; it may be associated with functional scoliosis as a result of poor posture.
4. Psychological distress may be a significant factor because of self-image issues, especially in adolescents.
5. Pectus deformities may need to be electively repaired before sternotomy for cardiac surgery.
D. Surgical repair
1. Typically supine position; arms may be tucked at both sides (open procedure) or abducted on arm boards (minimally invasive, or Nuss procedure).
2. Open (Ravitch) procedure
a. Transverse anterior chest wall incision (9)
b. Muscle and skin flaps
c. Resection of costochondral cartilage; each involved cartilage excised with preservation of the perichondrium
d. Sternal osteotomy(ies); cartilage grafts placed to maintain the new sternal position
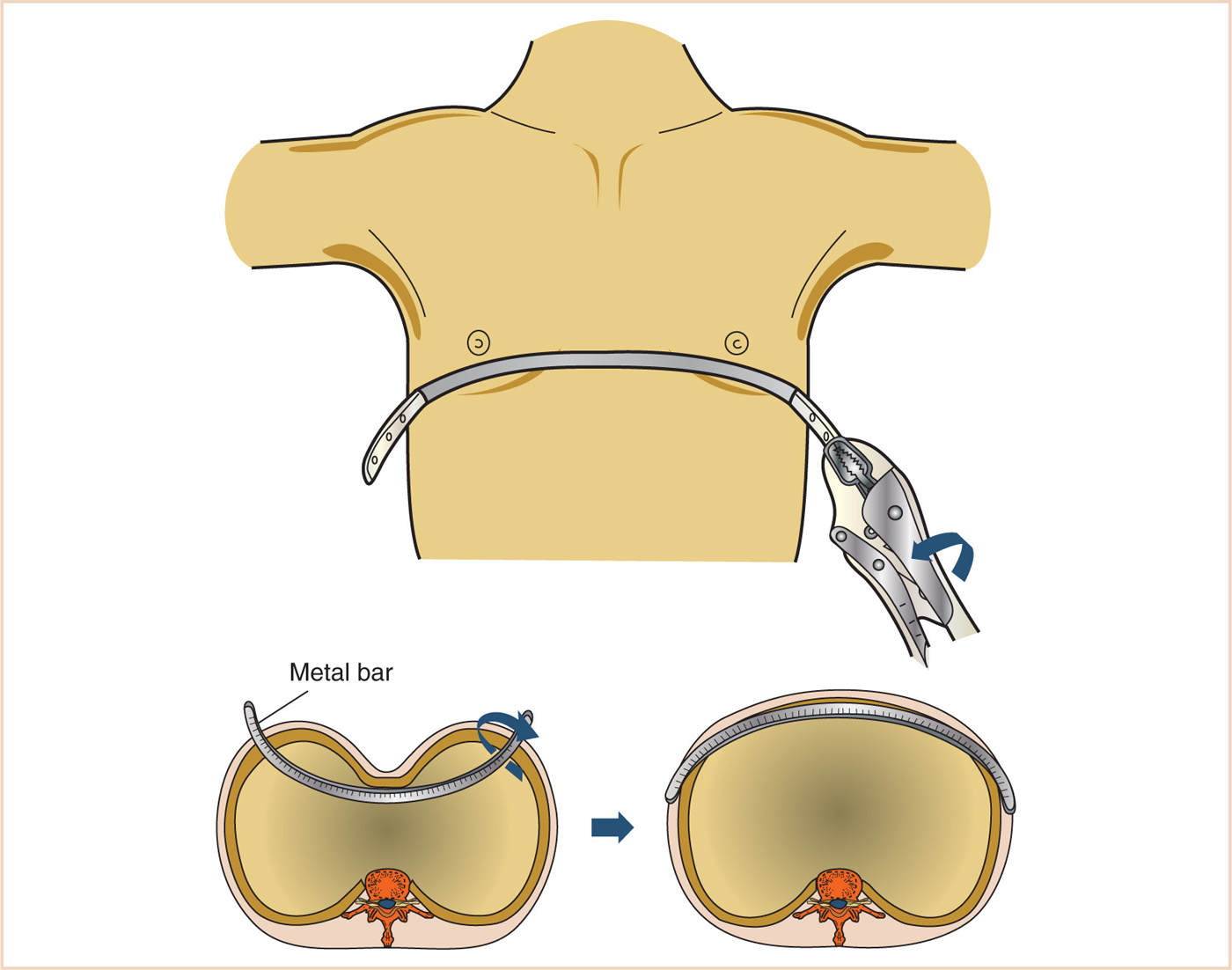
3. Minimally invasive (Nuss) procedure (see Fig. 17.9)
a. Small transverse bilateral skin incisions at midaxillary line (10–12)
b. Skin tunnel
c. Thoracoscope (typically right-sided) two intercostal spaces below the bar incisions
d. Insertion of pectus bar introducer and sternal lift (with thoracoscopic visualization)
e. Passing of umbilical tapes
f. Passing of previously formed pectus bar through the tunnel and retrosternal mediastinum, convexity posteriorly
g. Pectus bar rotation through 180 degrees so that concavity is now posterior
h. Bar fixation with stabilizers and sutures
E. Anesthesia issues
1. Readiness for surgery
a. Most patients are not significantly symptomatic. Echocardiography and pulmonary function testing may be useful for patients who are symptomatic with exercise.
b. Helpful imaging studies include posterior–anterior (PA) and lateral CXR and a chest computed tomographic (CT) scan.
2. Anesthesia goals
a. A general endotracheal anesthetic is required with or without a thoracic epidural for perioperative analgesia.
(1) Almost all adolescents, with sufficient anxiolysis, can have their thoracic epidurals placed while awake.
(2) Epidurals should remain in place for 3 to 4 days postoperatively.
b. Agitation and pain should be minimized from the time of anesthetic emergence and for the first 3 to 4 days postoperatively so that the risk of displacing the bar is minimal.
3. General anesthesia
a. Position: Supine
b. Typical surgical time: Open repair: 4.0 to 6.0 hours
c. Minimally invasive repair: 1.5 to 2.5 hours
4. Induction
a. Mask or IV induction with general endotracheal anesthesia
b. Muscle relaxation not necessary but may be a valuable adjunct for providing optimum surgical conditions and ensuring motionlessness
5. Equipment: Circle system breathing circuit
6. Monitoring: Standard noninvasive monitoring; bilateral axillary stethoscopy is helpful for clinical evaluation of pneumothorax.
7. Maintenance
a. Inhalation agent/balanced opioid technique if thoracic epidural is not utilized; otherwise, inhalation agent/segmental thoracic epidural blockade.
b. Neuromuscular blockade with nondepolarizing agent, if chosen.
c. If the pleural cavity is entered, it is usually decompressed by the application of large tidal breaths in Trendelenburg position. A chest tube may rarely have to be placed.
8. Emergence: Deep extubation should be strongly considered to avoid movement, emergence agitation, coughing, or bucking on endotracheal tube.
9. Perioperative
a. Chest wall analgesia through thoracic epidural or patient-controlled analgesia (PCA).
b. Sedation may be necessary as well to minimize patient movement, especially waist bending, twisting, logrolling, and sitting for the first few postoperative days.
10. Regional anesthesia
a. Epidural anesthesia, typically a thoracic catheter, is usually placed with sedation in adolescents. A segmental epidural blockade should be the goal, with approximately 2 mL of local anesthetic per segment to be blocked administered.
b. Thoracic paravertebral block, typically bilateral paravertebral catheters, is placed with ultrasound guidance.
DISORDER: Abdominal wall defects: umbilical hernia, patent omphalomesenteric duct, omphalocele, gastroschisis (13–15) (see Table 17.3)
A. Background
1. A range of abdominal wall defects may occur as a result of residual patency of the umbilicus and the omphalomesenteric duct, the most urgent of which are omphaloceles, sometimes confused in the neonatal period with gastroschisis.
2. Both defects in the abdominal wall of the neonate result in herniation of abdominal contents to varying degrees and are urgent neonatal surgical emergencies because of the threats of ischemia, bowel obstruction, and sepsis.
TABLE 17.3 Abdominal wall defects
Umbilical hernia | Outpouching of abdominal contents through a stretched umbilical ring defect, <4 cm in diameter, and always covered with skin. |
Patent omphalomesenteric duct | Enteroumbilical fistula—the entire duct may remain patent. Polyp—remnant of mucous membrane may persist at the umbilicus. Meckel diverticulum—proximal segment may fail to close entirely. Draining umbilical sinus tract—failure of distal segment to obliterate |
Omphalocele | Incomplete fetal return of the gut to the abdominal cavity, with exposed viscera covered by a thin, transparent membrane; the umbilical cord is at the apex. An epigastric omphalocele further associated with sternal and cardiac defects is known as the Pentalogy of Cantrell, and a hypogastric omphalocele may be further associated with exstrophy of the bladder and renal and genital anomalies. Omphalocele results from persistence of the body stalk and failure to develop the lateral wall. |
Gastroschisis | Herniation of bowel contents through a paramedian abdominal wall defect, usually to the right of the umbilicus. An isolated full-thickness abdominal wall defect, caused by failure in development of one of the lateral folds of the embryo. |
3. Less severe forms of residual patency of the omphalomesenteric duct are patent omphalomesenteric duct, omphalomesenteric duct cyst, and umbilical hernia.
B. Embryology/anatomy
1. By the third week of gestational age, the gastrointestinal (GI) tract is divided into the foregut, midgut, and hindgut.
2. The second portion of the duodenum to the proximal large bowel is derived from the midgut, which opens ventrally to the yolk sac, becoming the omphalomesenteric duct.
3. At 5 to 10 weeks of gestational age, the midgut, because of rapid growth, is extruded into the extraembryonic coelom (Fig. 17.6). This extruded gut rotates counterclockwise approximately 90 degrees about the omphalomesenteric (midgut) artery, which later becomes the superior mesenteric artery.
4. The midgut normally returns to the abdomen at the 10th week, where it continues its rotation another 180 degrees until the cecum and appendix become adherent to the posterior peritoneal wall in the right lower quadrant.
C. Physiologic considerations
1. Infection resulting from peritonitis, gut parenchymal disease, ischemia, and low perfusion with gut infarction
2. Fluid loss and profound dehydration
3. Heat loss and profound hypothermia
4. Hypoproteinemia, decreased plasma oncotic pressure, and hemoconcentration
5. Metabolic acidosis
6. GI obstruction with vomiting and distension
D. Surgical repair
1. Primary closure is ideal if the abdomen can accept the return of the viscera; however, a staged procedure may be required if the intra-abdominal space is inadequate.
a. The component separation technique is an alternate closure strategy for large abdominal wall defects consisting of separation of the skin and subcutaneous tissue, the external oblique, and the internal oblique, in order to achieve a tension-free layered closure with native tissue (1).
2. An omphalocele completely covered with an intact membrane may be less of an emergency than a gastroschisis (Table 17.4).
3. Skin flaps are elevated and the defect covered or Silastic or other mesh sheeting attached to the skin flap margins.
a. A pouch containing the herniated abdominal viscera is then created, which is gradually reduced into the abdomen by daily rolling.
b. This typically extends for 7 to 10 days.
TABLE 17.4 Omphalocele and gastroschisis
| Omphalocele | Gastroschisis |
Incidence (live births) | 1:3,000–1:10,000 | 1:30,000 |
Gender | Males > females | Male = females |
Associated anomalies | High association with other anomalies (50%–76%); | Rare |
| Cardiovascular 15%–25% (Tetralogy of Fallot = 33%) |
|
Prematurity incidence | 33% | 58% |
4. A gastrostomy may be placed in infants with gastroschisis; a central line for total parenteral nutrition (TPN) is typically placed for infants with an omphalocele.
E. Anesthesia issues
1. Readiness for surgery
a. Urgent, neonatal surgery
b. Medical stabilization for respiratory insufficiency
c. Medical stabilization for reduction of intravascular volume loss
d. Evaluation for additional anomalies
2. Anesthesia goals
a. Usual considerations for prematurity
b. Adequacy of intravascular volume and oxygen-carrying capacity
c. Adequacy of gas exchange
d. Maintenance of body temperature
e. Hematology support with red cells, fresh frozen plasma, and platelets, if needed
3. General anesthesia
a. Position: Supine
b. Typical surgical time: 3 hours
c. Induction
(1) An orogastric tube should be passed (if not already present) to drain the stomach of gastric contents.
(2) Before induction, intravascular volume should be reassessed.
(3) An awake intubation with direct laryngoscopy or a rapid sequence IV induction of anesthesia should be planned. Preoxygenation and cricoid pressure are required; then, a rapid sequence administration of sodium thiopental or propofol should be carried out.
(4) Muscle relaxation using succinylcholine with preceding or concomitantly administered atropine is typically utilized. Many now choose to administer rocuronium or mivacurium in doses designed to provide laryngeal relaxation within 60 to 90 seconds; however, significant oxygen desaturation may occur in these stressed neonates unless judicious, low peak inspiratory pressure and positive pressure breaths, “fluttering the lungs,” are delivered.
(5) Difficult airways should probably be approached with an awake intubation.
(6) Sizing the endotracheal tube is critically important; a tube with a low leak (i.e., <20 to 25 cm H2O) may provide an insufficient seal for positive pressure ventilation in the setting of high intra-abdominal pressures following reduction of the viscera into the abdomen.
CLINICAL PEARL Sizing the endotracheal tube is critically important; a tube with a low leak (i.e., <20 to 25 cm H2O) may provide an insufficient seal for positive pressure ventilation in the setting of high intra-abdominal pressures following reduction of the viscera into the abdomen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree