42 Sympathetic Neural Blockade
The sympathetic nervous system contains some of the afferent and efferent neural pathways necessary for generation, perpetuation, or treatment of certain clinical pain states. Sympathetic neural blockade may be useful in differentiating neuropathic pain processes that involve the sympathetic nervous system (sympathetically maintained/mediated pain—SMP) from those that do not (sympathetically independent pain—SIP). Most, but not all, SMP fulfills the clinical criteria for complex regional pain syndrome (CRPS) type 1 or type 2.1
The precise pathophysiology of SMP/CRPS is not fully understood, but loss of tonic sensory neuronal input associated with peripheral or other nerve injury produces chronically disordered information processing in the dorsolateral spinal cord with subsequently inappropriate responses to afferent sensory input and increased efferent sympathetic outflow.2–4 Typically, patients report severe burning discomfort or pain, or abnormal sensations, that may occur either spontaneously or secondary to even low-threshold stimuli. Physical findings, consistent with altered sympathetic tone, include erythema, edema, altered skin temperature, discoloration, and dystrophic changes of the skin, nails, and underlying bone and joints.2–5 Patients often exhibit guarding behaviors and physical findings consistent with disuse atrophy.
Other pain processes, such as visceral pain processes, may involve sympathetic afferents but may not produce a typical clinical composite of CRPS. Sympathetic nervous system involvement in visceral pain might be manifested as cutaneous hyperalgesia. Pain involving the sympathetic nervous system is accompanied by changes in central pain processing at spinal cord and higher levels. Functional magnetic resonance imaging (MRI) demonstrates changes in cerebral blood flow at thalamic and cortical levels in CRPS as well as in other chronic pain states,6,7 but the precise anatomic loci and molecular pharmacology of the sympathetic nervous system involvement remain ill-defined and generalized; changes are not limited to the painful side of the brain if the initial injury is unilateral.8
SMP is challenging to treat and it may be that earlier intervention increases the likelihood of successful treatment. The condition may be suspected when common limb disorders have been excluded and/or complaints of pain far exceed the nominal injury with or without signs or symptoms suggestive of altered sympathetic tone. SMP is a clinical diagnosis which may be supported by the presence of characteristic findings on physical examination, plain radiographs, triple-phase bone scan, thermogram, or significant pain relief with sympathetic blockade.2,4,5,9–11 MRI is often helpful in differentiating subacute or chronic nondisplaced fractures, pseudarthrosis, or neuroma, which are amenable to prompt surgical treatment, but manifest with a similar clinical constellation. Aggressive treatment protocols are required to obtain successful lasting pain relief and prevent chronic dystrophic changes. Local or regional sympathetic blockade is the cornerstone of treatment for SMP and is thought to help by interrupting and disorganizing the inappropriate sympathetic activity.3,4 Multimodal treatments appear to offer improved prospects for clinical success.12
Guanethidine, bretylium, or reserpine by intravenous or intravenous regional technique are not efficacious. Local anesthetics or analogs administered by oral, subcutaneous, intravenous, or intravenous regional techniques have also failed to demonstrate efficacy. These results may reflect the complexity of the underlying pathophysiology including, but not limited to, phenotypic shift of Aβ fibers to express substance P receptors, proliferation of ectopic α-adrenergic receptors, alterations in Na+ channel receptors or responsiveness to TNF-α which may accompany pathologic states. Changes noted in biologic markers, such as DRG calcitonin-gene-related peptide (CGRP) seen with neural blockade, do not correlate with treatment outcome. Specific effects of sympathetic neural blockade on receptors for glutamine, NMDA, TRK, other vanilloids, or substance P are not known. Although physical therapy may be beneficial for analgesic modalities, for reduction of edema, and for promotion of active mobilization and reconditioning of involved extremities, common psychological comorbid conditions include anxiety, depression, and emergence of clinical personality disorders, similar to those seen in other patients with chronic pain. Recalcitrant CRPS unresponsive to traditional techniques may respond to spinal cord stimulation.13 Surgical sympathectomy may be considered for refractory circumstances, but success is far from assured and the duration of improvement is variable.
In the past decade, the putative role of the sympathetic nervous system in clinical pain has become more subtle and more complex with emerging neuroanatomic evidence for dual sympathetic and somatic innervation of many structures including cervical and lumbar zygapophysial joint capsules. Curiously, however, painful zygapophysial spinal joints can be successfully treated with thermal radiofrequency neurolysis (of the medial branches) and the author is unaware of any proven case of zygapophysial joint pain resolved by sympathetic neural blockade. RF lesioning of the medial branch does not, when properly conducted, produce CRPS.14,15 The role of dual sympathetic and somatic innervation of the lumbar intervertebral disc has provided a putative basis for treatment of discogenic pain by sympathetic neural blockade or by RF lesioning of the gray rami communicantes by RF thermolysis.16
Sympathetic nerve block is often used in a diagnostic capacity for interruption of afferent or efferent neural pathways. Results of neural blockade generally, but do not always, correlate with outcomes from repetitive neural blockade, surgical sympathectomy, and percutaneous chemical, cryotherapeutic, or thermolytic (RF lesioning) procedures. Limb or visceral pain, and in particular CRPS, which responds only transiently to sympathetic block may be improved with spinal cord stimulation.17
In some situations, use of targets anatomically adjacent to sympathetic nerve structures, but without image guidance, may suggest clinical efficacy,18 but more highly directed treatments may not be supportive.19
Stellate Ganglion Block (SGB)
Anatomy
The ganglion is typically about 2.5 cm in length and is located at the root of the C7 transverse process; it lies anterolateral to the longus colli muscle. The ganglion is anterior to the transverse process in the sagittal plane and posterior to the apical pleura which rises above the level of the first rib, posing a hazard of pneumothorax for anterior approaches at the C7 level or below. The carotid artery is anterior to the ganglion and the vertebral artery is anterolateral inferiorly, subsequently crossing over the sympathetic chain as it ascends to enter the foramen transversarium at C6 or above in 95% of individuals.20,21 At C6, the inferior thyroid artery is also anterior to the ganglion. Sympathetic nerve branches from the stellate ganglion extend to the brachial plexus, subclavian and vertebral arteries as well as the brachiocephalic trunk.22 Cardiac sympathetic nerves arise from the ganglion as does the vertebral nerve, which provides sympathetic innervation of the fibrous capsules of the zygapophysial and intervertebral joints and meningeal structures.15
Merged inferior cervical and thoracic ganglia are present in approximately 85% of patients, but stellate ganglion block (SGB) may fail to fully interrupt the sympathetic neural innervation of the head, neck, upper extremity, and upper thorax for several reasons. Although limited spread of local anesthetic may potentially fail to deliver agent onto disunited sympathetic ganglia, the presence of these sympathetic ganglia within the same fascial plane makes this unlikely.23 The distribution of radiographic contrast agent or dye in cadaveric studies demonstrates that injected volumes of 5 to 10 mL are routinely adequate to envelop the ganglion and may extend as far caudad as the T2 vertebral level.24,25 The nerve(s) of Kuntz are ascending ramus communicans branches originating at T2, T3, or T4 in 66% to 80% of individuals.26 These nerves are located approximately 7 mm from the sympathetic chain and provide an alternate pathway for sympathetic nerve fibers to bypass the sympathetic chain and enter directly into the first intercostal nerve or into the T1 nerve root. These Kuntz nerve fibers are not routinely bathed in local anesthetic agent during SGB and increased volumes of local anesthetic do not increase efficacy, but may produce undesirable spinal nerve root or brachial plexus block as well as unpredictable contralateral spread.
Indications
Twentieth century medical literature records dozens of reported clinical series wherein SGB was performed for a variety of clinical indications, all incorporating diagnostic or therapeutic applications where putative interruption of sympathetic afferent or efferent function of the head, neck, upper extremity, or thorax was postulated as useful. Present indications for SGB include the following27:
Contraindications
Pregnancy, although contraindicating exposure to ionizing radiation and fluoroscopy, may not prevent performance of SGB with ultrasound guidance; however, details of the ultrasound technique and potential obstetric issues are beyond the scope of this chapter.29 Narrow angle glaucoma may be considered a contraindication owing to expectable miosis produced by the loss of sympathetic tone occurring with SGB.
Recent myocardial infarction was formerly considered to represent a relative contraindication to SGB owing to risk of dysrhythmia. Newer evidence, however, supports sympathetic nerve sprouting and spontaneous increased sympathetic activity, which may follow myocardial infarction as a mechanism for sudden cardiac death.30,31 A small experimental study of bilateral SGB in a rat coronary ischemia model demonstrated reduced ST segment elevations and substantially reduced incidence of ventricular tachycardia or fibrillation when compared to controls.32
Complications
These should not be considered as complications but as expected side effects.
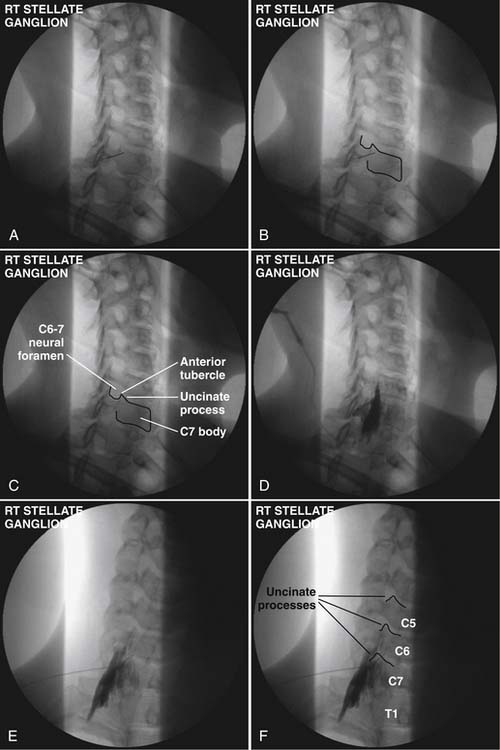
Technique (Fig. 42-1)
A 5 mL volume of local anesthetic is sufficient for adequate SGB performed under fluoroscopy, CT,33 MRI, or ultrasound imaging.29 Evidence from cadaveric and in vivo studies suggests that larger volumes (i.e., 20 mL) are neither necessary nor additionally efficacious because they often produce unwanted effects. Use of a 5 mL volume of bupivacaine 0.125%, bupivacaine 0.25%, or ropivacaine 0.2% minimizes risk of systemic local anesthetic toxicity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree