Fig. 7.1
Multiport trocar placement
Any inflammatory adhesions to surrounding viscera are then cleared, and medial and lateral hepatic attachments of the gallbladder are released. The hilum is dissected clear of adherent tissue and adhesions using a blunt dissector and electrocautery hook. In the acutely inflamed gallbladder, the expected anatomy may be obscured by dense adhesions, edematous tissues, and the limited view of a laparoscope. Resultant misidentification of structures can lead to vascular or biliary injury. A technique developed by Strasberg in 1995 called the “critical view of safety” has been so effective in preventing intraoperative injury to surrounding structures that it is now incorporated into the standard dissection [4]. The triangle of Calot must first be skeletonized of adherent tissue. The gallbladder hilum is separated from the cystic plate. The critical view is then achieved with visualization of two (and only two) discrete structures entering the gallbladder: the cystic artery and the cystic duct (Fig. 7.2). If all of these criteria are met, then the surgeon can confidently proceed with transection of the cystic duct and the cystic artery.
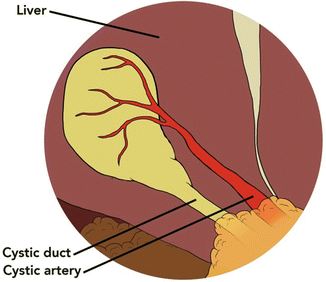

Fig. 7.2
Critical view of safety: two distinct structures are seen entering the gallbladder
After dissection of the cystic triangle, the cystic duct is clipped on the gallbladder side to minimize the potential for stones to migrate into the bile ducts during manipulation. At this point, if a cholangiogram is indicated or desired, the cystic duct is cannulated using a cholangiocatheter. Water soluble contrast dye can be injected and fluoroscopy performed to evaluate the biliary system. The cystic duct and cystic artery are then clipped proximally and distally and divided. Electrocautery hook is used to separate the gallbladder from the liver bed, moving from the hilum to the fundus.
If dissection of Calot’s triangle proves difficult, particularly in cases where gallbladder anatomy is obscuring the cystic triangle such as in Mirizzi’s syndrome, a dome-down approach can be used. Similar to the technique used in open cholecystectomy, the cystic plate is divided superiorly at the fundus with dissection proceeding toward the ductal structures. Some experts claim a lower rate of converting to an open procedure and faster recovery with this approach [5–7].
In gangrenous or severely inflamed gallbladders, standard clips may be inadequate to control the cystic duct. Laparoscopic staplers can be used to divide either the cystic duct proper or the distal gallbladder. If a remnant gallbladder is left, the surgeon and patient must be aware of the risk of recurrence or incomplete control of infection. Alternatively, the cystic duct can be suture ligated using intracorporeal suture. Finally, an endoloop ligature can be used to control the proximal cystic duct.
Once successfully freed, the gallbladder is removed through the umbilical port using a specimen bag. The trocars are removed under direct supervision, and port sites are closed and dressed according to surgeon preference.
Postoperatively, the patient can tolerate a clear liquid diet, which is advanced as tolerated to a full diet. Pain control can be achieved with oral analgesics, and no further antibiotics are required. Even in the case of acute cholecystitis, the patient is routinely discharged on the first postoperative day.
Role of Intraoperative Cholangiogram and Ultrasonography
If indicated, intraoperative cholangiogram (IOC) is performed after identification of the cystic duct. The duct is clipped on the gallbladder side and scissors are used to create a small ductotomy. A small, 4–5 Fr catheter is introduced into the peritoneal cavity via angiocath beside a subcostal port and guided into the cystic duct lumen through this defect. Saline is used to prime the catheter prior to insertion to prevent accumulation of air bubbles. Images are obtained with a portable X-ray machine present in the operating room. These images are used to identify ductal stones or an enlarged common bile duct. If choledocholithiasis is identified, the surgeon may proceed with a common bile duct exploration laparoscopically or via laparotomy. It is also routine in many centers to complete the laparoscopic cholecystectomy as planned; the patient then undergoes postoperative endoscopic sphincterotomy and stone extraction. The cholangiogram may also be used clarify anatomy and ensure appropriate identification of structures.
IOC can be performed either selectively or routinely during laparoscopic cholecystectomy to evaluate for ductal stones. Selective use is indicated with a preoperative history of jaundice, pancreatitis, or enlarged common bile duct on imaging. Some surgeons choose to routinely perform IOC to decrease the incidence of occult retained stones and to confirm biliary anatomy with the intention of protecting against injury to the extrahepatic biliary tract.
However, the use of IOC for anatomic delineation is controversial. Large population-based studies from Sweden, Australia, and the USA have found IOC to be protective against biliary injury [8–10]. However, a recent multicenter retrospective analysis of almost 93,000 patients undergoing cholecystectomy showed no statistical difference in the number of common bile duct injuries when the data was controlled for confounding variables [11]. There is data to suggest that cholangiogram can successfully be used to diagnose bile duct injury at the time of operation [12, 13]. One study quotes a sensitivity of 79 % and specificity of 100 % for intraoperative diagnosis of common bile duct injury when IOC is routinely performed [12]. While IOC cannot be relied upon to prevent injury, it may confirm anatomy prior to transection of critical structure and allow for immediate diagnosis and repair of injury at the time of the primary operation.
As an alternative to cholangiogram, laparoscopic ultrasonography has shown initial success for defining difficult anatomy [14]. In this technique, a 10 mm ultrasound probe is inserted through either the periumbilical or epigastric port. A transverse image is obtained of the portal triad in the hepatoduodenal ligament, and the probe is manipulated to visualize the extrahepatic biliary tract. Doppler signal can be used to differentiate vascular structures from biliary structures and can also identify ductal stones with an experienced operator. Despite these reports of success, laparoscopic ultrasound remains an uncommonly used technique.
Single Incision Approach
Single incision laparoscopic cholecystectomy (SILC) was introduced in 1995 by Navarra, 10 years following the introduction of the multiport cholecystectomy [15]. Surgical technique for SILC is not standardized and is not as popular as the traditional multiport operation. This technique can be performed using either one large access platform or a single skin incision with the insertion of multiple ports.
For single incision surgery, the patient is prepped in an identical manner as for a multiport operation. A 2.5 cm incision is made in the umbilicus and extended into the peritoneum. A platform device is inserted, and 4–5 trocars are placed within the platform (Fig. 7.3). The abdomen is insufflated, and the subsequent dissection is carried out in the same manner as described for multiport cholecystectomy. The primary difference in surgical technique is that electrocautery is held in the left hand and retraction is supplied by the right to prevent excessive crossing of instruments. The postoperative course is as previously described.
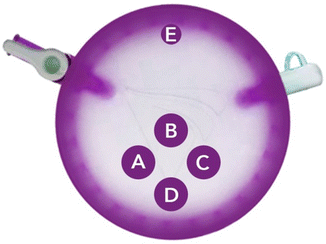

Fig. 7.3
Trocar placement in Single Incision platform. (a) Camera. (b) Dissector. (c, d) Retractors. (e) Angiocath insertion for cholangiogram
No large-scale randomized trials have been conducted to compare SILC to standard laparoscopic cholecystectomy. Initial meta-analyses of small, randomized trials and case series showed an increase in operative time and complications, especially biliary tract injuries [16–18]. However, a current meta-analysis that included almost 7500 patients found no difference in overall rates of complications or biliary spillage [19]. Routine cholangiogram can be successfully performed with SILC [20]. Additionally, a multicenter randomized trial of 200 patients with biliary colic found an increase in rates of hernia but no increase in overall or serious adverse events, with an improved self-reported cosmetic outcome [21].
As the surgical community has gained familiarity with the SILC technique, it is now being applied to patients with acute cholecystitis. There are series that report safety of SILC in acutely inflamed gallbladders when performed by experienced surgeons [22]. In a single-surgeon prospective series that has been accepted for publication from our institution, SILC was completed on all patients with an indication for cholecystectomy regardless of pathology. There was a slightly increased rate of conversion to multiport laparoscopy in acute cholecystitis when compared to noninflammatory disease but no increase in the rate of conversion to open cholecystectomy or in the number of serious complications.
Ultrasonic Dissection
The use of ultrasonic dissection devices such as the Harmonic (Ethicon) in laparoscopic cholecystectomy are gaining popularity. Ultrasonic scalpels take advantage of ultrasound waves at a frequency that cause coagulation, cutting, and cavitation of tissues with minimal spread of injury [23]. They have the advantage of eliminating thermal injuries to surrounding structures that are seen with traditional electrocautery and can also be used to ligate the cystic artery and duct without the use of suture or clips [24]. Proponents cite shorter operating times, lower risk of bile leak, and faster recovery [25]. However, ultrasonic dissectors require special training and have a higher cost than traditional electrocautery and are not yet routinely used in laparoscopic cholecystectomy.
Salvage Operations
In some cases, anatomy is unfavorable or a patient is too unstable to proceed with planned laparoscopic operation. In this situation, the surgeon has the option of converting to an open procedure or performing a salvage operation. Salvage operations include subtotal cholecystectomy and cholecystostomy tube placement. They are used to avoid futile and dangerous attempts to dissect Calot’s triangle in cases of severe inflammation, empyema, or fibrosis in which the anatomy is obscured [26, 27]. If performed laparoscopically, these procedures can avoid conversion to an open procedure and the associated morbidity [28].
Subtotal cholecystectomy (Fig. 7.4) is undertaken after attempts at traditional cholecystectomy have failed. The anterior wall of the gallbladder is opened, and bile and stones are suctioned out. If the cystic duct can be identified, it is suture ligated. If the cystic duct cannot be identified, it can be left to fibrose with a drain in place. The anterior gallbladder is resected leaving only the posterior wall adherent to the hepatic plate. The remaining mucosa is cauterized and a drain is left in the gallbladder fossa. Potential complications include increased risk of intra-abdominal abscess and surgical site infection, biliary leak, and the potential for undiagnosed gallbladder cancer in the ablated mucosa. Nonetheless, at least one recent comparison between subtotal and total cholecystectomy showed no increase in the number of postoperative complications, and no retained stones or recurrent acute cholecystitis at 42 months [29].
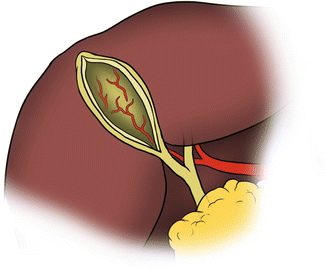

Fig. 7.4
Subtotal cholecystectomy. The anterior gallbladder is resected and the remaining mucosa ablated
In cases of severe acute cholecystitis and intraoperative hemodynamic instability, the cholecystectomy may be aborted and a cholecystostomy drain placed across the wall of the gallbladder. Like percutaneous drains, this is a temporizing measure to drain the gallbladder to treat acute infection. An interval cholecystectomy is indicated once the patient has appropriately stabilized and infection is resolved, typically at a minimum of 6 weeks from the initial hospitalization.
Open Cholecystectomy
Early criticisms of laparoscopic cholecystectomy cited high complication rates when compared to the traditional open approach. In the past, early conversion to laparotomy was advocated in cases in which anatomy was obscured by inflammation. Operative techniques have been refined since that time to improve outcomes and exposure, and now the overall rates of surgical complications in laparoscopic cholecystectomy are superior to that of the open procedure [30]. In certain cases, however, it still remains necessary to convert from laparoscopic to open cholecystectomy. Indications include intolerance of pneumoperitoneum, severe inflammation or otherwise limited view, uncontrollable bleeding, malignancy, or suspected or confirmed biliary injury.
An open cholecystectomy is performed via an upper midline or right subcostal incision 2–3 cm inferior to the costal margin. The peritoneum overlying the gallbladder is incised 1 cm away from the liver edge. Adhesions between the inflamed gallbladder and adjacent viscera, most commonly duodenum and transverse colon, are identified and sharply divided. Dissection along the avascular plane between the gallbladder and the hepatic bed is carried from the fundus inferiorly using electrocautery. The cystic artery and duct are identified and individually clipped or suture ligated. The specimen is removed and the fascia and skin are closed in the usual manner.
As surgeons and trainees are gaining more experience with laparoscopy and techniques are becoming more sophisticated, many argue that there are few strict indications for conversion to open cholecystectomy. A Dutch retrospective review analyzed the severity of biliary duct injuries before and after conversion and found that of all injuries that occurred, open procedures produced more severe biliary injuries [31]. These authors propose that the increased severity of injury occurs because of inadequate training in open cholecystectomy in young surgeons.
Complications
Complications of cholecystectomy include bile duct injury, bile leak, and retained stones. The associated clinical syndromes vary widely in timing and severity of presentation.
Bile Duct Injury and Bile Leak
Bile duct injury (BDI) is the most feared complication of cholecystectomy and is associated with significant morbidity and mortality. As techniques in laparoscopy have improved, the incidence of BDI has decreased dramatically. The exact incidence is unknown, but in recent laparoscopic series is reported to be as low as 0.1–0.3 % [32, 33].
Risk factors for biliary injury include obesity, advanced age, male sex, dense adhesions, and aberrant anatomy [34]. Multiple classification systems have been introduced in an attempt to guide management of BDI [35–37]. Perhaps most commonly used is the Strasberg-Bismuth classification system (Fig. 7.5). In this system, Class A injuries consist of a bile leak from the cystic duct or from a small liver duct in the gallbladder fossa. Class B injuries represent transection of an accessory duct, usually an aberrant right hepatic duct. Class C are bile leaks from an accessory duct. Class D is partial transection anywhere along the bile duct system, and Class E is a complete transection of the bile duct, subdivided based on location [37]. The type of injury helps determine the most appropriate method of repair.
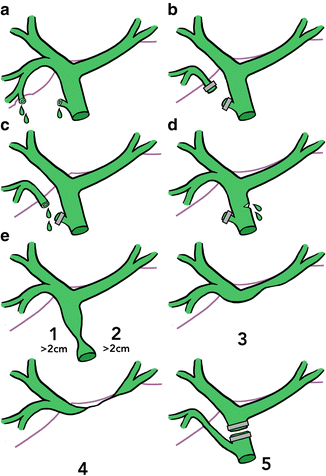

Fig. 7.5




Strasberg-Bismuth classification of bile duct injuries. (a) Cystic duct stump leak. (b) Transection of accessory duct. (c) Accessory duct leak. (d) Partial transection of any bile duct. (e) Complete transection of common bile duct, subdivided based on location of injury (1–5).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








