Medications:
Gabapentin
100 mg three times a day
Tramadol
50 mg every 6 h if needed
Hydrochlorothiazide
12.5 mg twice a day
Metoprolol
25 mg once a day
Losartan
50 mg once a day
Pantoprazole
40 mg twice a day
Crestor
10 mg twice a day
Allergies:
NKDA
Past Medical History:
Cardiac:
HTN, Hyperlipidemia
Pulmonary:
Obstructive sleep apnea (OSA)
GI:
Gastroesophageal reflux disease
Other: Chronic Pain
Physical Exam:
Vital signs:
BP 145/71, HR 56, RR 18, SpO2 95%, Weight 89.9 kg, Height 1.72 meters, BMI 30.4, obesity class 1
Airway:
Mallampati 3, limited neck extension and flexion due to pain
Neuromuscular:
Deltoid muscle weakness, positive hoffmann’s bilaterally, spastic gait while walking, weakness of lower legs
EKG:
NSR, first-degree AV block, non-specific ST-T changes
Otherwise:
Insignificant
- 1.
Describe the anatomy of the spinal column and spinal cord
The spinal column is comprised of 33 vertebrae: 7 cervical vertebrae, 12 thoracic vertebrae, 5 lumbar vertebrae, sacrum (5 fused vertebrae), and coccyx (4 fused vertebrae). Each vertebra, except C1, has a vertebral body, bilateral pedicles, bilateral lamina, bilateral transverse processes, a spinous process, and 4 articular processes. Vertebrae connect to each other via superior and inferior facets and inter-vertebrae disks. Two posterior lamina, two lateral pedicles and vertebra body anteriorly form the vertebral canal where the spinal cord lies. C1 (Atlas) lacks vertebrae body and consists of an anterior arch and tubercle, posterior arches, spinous process, and lateral masses. C2 (Axis) has a strong odontoid process that articulates with C1.
The spinal cord is a continuation of the medulla at the foramen magna and extends to the conus medullaris at the first or second lumbar vertebra in the vertebral canal in adults. On a cross-sectioned view, the cord is composed of gray matter and white matter. The gray matter resembles the shape of the letter H, surrounds the central canal, and contains cell bodies of neurons. It is divided into four main columns: the dorsal horn, intermediate horn, ventral horn, and lateral horn (see Fig. 18.1) [1]. The white matter contains myelinated and unmyelinated nerve fibers that carry information through the cord. The white matter is divided into the dorsal column (or funiculus), lateral column and ventral column. The cord has four regions (cervical, thoracic, lumbar and sacral) and two bulges at the cervical and lumbar region. The dorsal roots and ventral roots join together to form 31 pairs of spinal nerves that exit from spinal foramen. The spinal cord is surrounded with three meninges: pia, arachnoid, and dura.
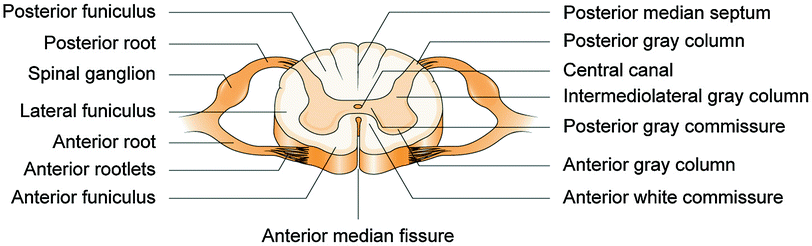

Fig. 18.1
Anatomy of spinal cord (cross-section view). Reproduced from Stier et al. [1, Figs. 20–15], with permission from Elsevier
- 2.
Which arteries supply blood to the spinal cord?
The anterior spinal artery, two posterior spinal arteries, and radicular arteries are the main arteries that supply blood to the spinal cord. The vertebral arteries at the medulla level branch off to form the anterior spinal artery, which supplies the anterior two-thirds of the cord, while the posterior spinal arteries arising from either vertebral arteries or posterior inferior cerebellar arteries on the same side supply the posterior one-third. The radicular arteries that originate from the segmental arteries of aorta further augment the blood supply to spinal cord. The Artery of Adamkiewicz is the largest segmental feeder in the thoracolumbar region. Injury to those segmental arteries can cause anterior spinal cord ischemia, resulting in paralysis.
- 3.
How is blood flow to the spinal cord regulated?
The spinal cord blood flow (SCBF) is autoregulated and kept relatively constant. Average SCBF is about 60 ml/100 g/min, but the blood flow to gray matter is four times higher than in white matter. SCBF is well maintained at the mean arterial blood pressure (MAP) between 60 and 120 mmHg [1]. Blood pressure that is lower than the autoregulation lower limit causes ischemia to the spinal cord. Hypoxia and hypercarbia increase SCBF, while hypocarbia reduces SCBF.
- 4.
What are the indications for spine surgery?
Spine surgery is indicated for following conditions:
- (1)
Degenerative spine pathology resulting neurological dysfunction and pain, such as spondylosis, spondylotic myelopathy, spinal stenosis, spondylolithesis, and disk herniation;
- (2)
Spinal structure instability requiring stabilization, such as vertebra fracture due to trauma or other pathology;
- (3)
Infection of spine, such as spinal abscesses and tuberculosis;
- (4)
Spinal deformity, such as scoliosis;
- (5)
Spinal tumors, such as meningiomas and intraspinal cord tumors;
- (6)
Spinal hematoma that compresses the spinal cord or nerve roots;
- (7)
Inflammatory diseases, such as rheumatoid arthritis and ankylosing spondylitis.
- 5.
What is cervical spondylotic myelopathy?
Cervical spondylotic myelopathy (CSM) is a syndrome produced by central spinal canal stenosis and compression of the spinal cord due to cervical spondylosis, a progressive degenerative process affecting the vertebral body and disk. CSM is the most common cause of myelopathy in older adults.
The common possible manifestations are summarized [2] as following:
- (1)
pain in the neck and subscapular or shoulder that radiates to the arms;
- (2)
numbness, or paresthesia, in the arms;
- (3)
gait disturbance characterized by a spastic, scissoring quality;
- (4)
sensory deficits related to the dorsal column;
- (5)
weakness in the lower extremities with upper motor neuron characteristics (increased reflexes and muscle tone, and the presence of the Babinski sign);
- (6)
lower motor neuron findings (weakness in the arms and hands);
- (7)
bladder dysfunction;
- (8)
Lhermitte’s sign; an electric shock-like sensation in the neck that radiates down to the spine and arms, produced by forward flexion of the neck.
Magnetic Resonance Imaging is used to confirm the diagnosis. The Nurick grading system [3] classifies the severity of CSM from the least severe, grade 1, to the most severe, grade 5 based on gait abnormality (see Table 18.1).
Table 18.1
Nurick classification of cervical myelopathy based on gait abnormality
Grade | Gait abnormality |
|---|---|
1 | Spinal cord disease with no problem working |
2 | Slight difficulty walking and cannot work full time |
3 | Difficulty walking and cannot work full time |
4 | Can only walk with help of frame walker |
5 | Chair-bound or bedridden |
- 6.
What is central cord syndrome?
Central Cord Syndrome (CCS) is the most common incomplete spinal cord injury and is characterized by a disproportionately greater motor impairment in the upper extremities than in lower extremities, bladder dysfunction, and various sensory losses below the level of injury. The mechanism involves the compression of the cord by osteophytes and infolded ligamentum flavum. Patients with CSM can suffer CCS after minor neck injury without evidence of spinal fracture. Hyperextension of neck should be avoided during direct laryngoscope in patients with CSM to prevent neurological damage.
- 7.
What is the anterior cord syndrome? What is the Brown-Sequard syndrome?
Anterior cord syndrome (ACS) results from injury to the anterior spinal artery or compression of the anterior cord. It is characterized by variable motor impairment, pain, and temperature sensation impairment with preservation of proprioception below the level of injury.
Brown-Sequard syndrome an incomplete spinal cord injury and results from hemisection of spinal cord. It is manifested as ipsilateral loss of motor and proprioception with contralateral loss of pain and temperature sensation. It usually occurs after penetrating trauma.
- 8.
What is spinal shock?
Spinal shock is a neurogenic shock caused by an interruption of sympathetic output from the spinal cord and unopposed parasympathetic activity after acute spinal cord injury (SCI). The severity of spinal shock is related to the severity and completeness of SCI. Loss of sympathetic activity below the level of injury results vasodilation and decrease in venous return, leading to hypotension. Bradycardia can occur if the SCI is above T6 level. Treatment includes fluid therapy and the use of vasopressors to support blood pressure in order to maintain MAP > 85 mmHg for the first week following the initial injury.
- 9.
What is the American Spinal Injury Association (ASIA) impairment scale?
The ASIA impairment scale is used to define the severity of a spinal cord injury. It combines the sensory and motor deficits with completeness of the injury to classify the injury into five grades from Grade A to grade E. The ASIA impairment scale evaluates and scores 10 key muscle groups and 28 dermatomes for both light touch and pin prick.
Grade A: Complete injury; no motor or sensory function is preserved in sacral segments S4-S5.
Grade B: Sensory incomplete injury; sensation is preserved without motor function below the neurological level, and includes S4-S5.
Grade C: Motor incomplete injury; motor function is preserved below the neurological level, and more than half of the key muscle functions below the neurological level of injury have a muscle grade <3.
Grade D: Motor function is preserved below the neurological level; more than half of the key muscle functions below the neurological level of injury have a muscle grade ≥3.
Grade E: Normal.
- 10.
What is the new neurological deficit (NND) rate for spinal surgery?
Spinal surgery poses potential risks to the spinal cord, nerve roots, caudal equina, and peripheral nerves. Although the overall incidence of NND is small, the consequence of neurological complications, such as spinal cord paralysis, could be disastrous for both patient and family. Revision surgery, spinal fusion, use of implants, and surgical approach may affect the rate of NND. The most recent data from the Scoliosis Research Society shows that the overall rates of new nerve roots, cauda equina, and spinal cord deficits for 108,419 patients were 0.61, 0.07, and 0.27% [4].
- 11.
What is the mortality rate of spine surgery?
The mortality of spinal surgery is low and varies depending on the location of the procedure. Lumbar spine (range from 0.07 to 0.52%) and cervical spine surgery (range from 0.1 to 0.8%) carry lower mortality compared to thoracic spine surgery (range from 0.3 to 7.4%) [5]. Recent studies from the Scoliosis Research Society Morbidity and Mortality database showed that the overall mortality rate is 1.8–1.9 per 1000 spinal deformity procedures [6, 7]. However, mortality increases in patients over 60 years of age. High ASA scores, fusion and implants are associated with an increased mortality rate. The main causes are respiratory (respiratory failure, pneumonia, pulmonary embolism, etc.), cardiac (cardiac failure, myocardial infarction, or cardiac arrest), sepsis, multi-system organ failure, stroke, and blood loss. Although mortality due to intraoperative blood loss accounted for 4% of all mortality, it can be prevented if careful planning and intraoperative management are implemented.
- 12.
What are the goals of intraoperative neuromonitoring (IONM) during spine surgery?
There are three goals of IONM during spine surgery. The principle goal of IONM is to prevent injury to the spinal cord and nerve roots by way of surgical manipulation and instrumentation. Secondly, IONM is used to prevent ischemia due to hypoperfusion to the spinal cord. The third goal is to prevent peripheral nerve injury by way of inappropriate positioning.
- 13.
What is the somatosensory-evoked potential (SSEP)? How does SSEP monitoring prevent spinal cord injury during spinal surgery?
SSEPs are electric responses from peripheral nerve stimulations. It was introduced in the 1980s and is indicated for monitoring spinal cord integrity in scoliosis correction, spinal cord tumor, spinal decompression, and instrumentation surgeries. SSEPs can be recorded along sensory pathways. Cortical SSEPs are recorded from the electrodes placed on scalp according to the international 10–20 system. The most common peripheral stimulating sites are the ulnar or medium nerve for upper extremity SSEPs, and the posterior tibia nerve or common peroneal nerve for lower extremity SSEPs. SSEPs voltage is very small compared to EKG and EEG; it requires averages to obtain high quality waveforms for monitoring by reducing 60 Hz interferences. SSEP is characterized as amplitude and latency that are affected by many factors. SSEP waveforms P14 and N20 from upper extremity and N34 and P37 from lower extremity are commonly used for monitoring. The baseline SSEPs must be obtained before the surgical incision.
SSEPs are primarily mediated by the dorsal column-medial lemniscal system. The anatomy of this sensory pathway includes peripheral nerves, first-order neurons in the dorsal ganglion, second-order neurons with nucleus gracilis and cuneatus, third-order neurons in the thalamus, and sensory cortex. Peripheral nerves enter the dorsal root ganglion where primary sensory neurons receive sensory input. The central axons of sensory neurons ascend ipsilaterally within the fasciculus gracilis and fasciculus cuneatus to the caudal medulla where the neuron fibers synapse with second-order neurons within the nucleus cuneatus and nucleus gracilis. The axons of second-order neurons decussate and ascend as medial lemniscus to third-order neurons in the ventral posterior lateral nucleus of the thalamus. The third-order neurons in the thalamus project axons to the primary somatosensory cortex in the contralateral posterior gyrus. When the peripheral nerves are stimulated, action potentials propagate along the pathway and generate the SSEPs that can be recorded. Any disruption to the sensory pathway can lead to loss of SSEP production. Surgical manipulation and instrumentation during spinal surgery could result in spinal cord and nerve root injury. SSEP monitoring will alert both the surgeons and anesthesiologists for any impending spinal cord injury, thus allowing surgeons to correct reversible causes in time, while the anesthesiologists affirm physiological and anesthesia stability.
- 14.
What are the anesthetic effects on SSEP recording?
Anesthetics affect cortical SSEPs but have minimal effect on subcortical SSEPs. The deeper the anesthesia is, the more SSEPs are suppressed. It is very important to keep anesthetic level steady state while SSEP’s are recorded.
- (1)
Halogenated inhalational anesthetics such as isoflurane, sevoflurane and desflurane depress the amplitude of SSEP’s and prolong latency of SSEPs in dose dependent fashion. Those agents affect cortical SSEPs more than subcortical SSEP’s because more synapses are involved in generating cortical SSEPs. In our practice, we use less than 1 MAC of inhalational agent, and the recordings of SSEPs are usually adequate for monitoring.
- (2)
Nitrous oxide (N2O) produces an increase in SSEP’s latency and decrease in amplitude of SSEPs. It affects cortical SSEPs more than other halogenated agents at equal potency. N2O use should be avoided if the cortical SSEPs are desired for monitoring.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





