Spinal Pain and the Role of Neural Blockade
James P. Rathmell
Carlos A. Pino
Shihab Ahmed
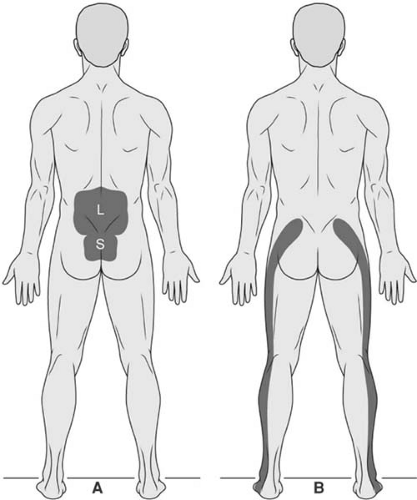
Low back pain is a nonspecific term, typically used to refer to pain that is centered over the lumbosacral junction. To be more precise in our approach to diagnosis and treatment, it is important to differentiate pain that is primarily over the axis of the spinal column from pain that is primarily referred to the leg (Fig. 44-1). Lumbar spinal pain is pain inferior to the inferior aspect of the 12th thoracic spinous process and superior aspect of the 1st sacral spinous process (1). Sacral spinal pain is pain inferior to the first sacral spinous process and superior to the sacrococcygeal joint (1). Lumbosacral spinal pain is pain in either or both of these regions and constitutes what is commonly referred to as “low back pain.” A subset of patients will present with pain that is predominantly localized within the leg and is commonly called “sciatica,” because of its distribution in the area innervated by the sciatic nerve; the proper term for this type of pain is radicular pain, and this pain is evoked by stimulation of the nerve roots or the dorsal root ganglion of a spinal nerve.
Pain in the thoracic and cervical regions is less common than low back pain, and our understanding of treatment is less well developed. Nonetheless, cervical and thoracic pain are common. Cervical spinal pain is pain inferior to the occiput, extending inferiorly to the inferior aspect of the 7th cervical spinous process. Thoracic spinal pain is pain anywhere between the 1st and 12th thoracic spinous processes. As described for lumbosacral pain, when approaching patients with spinal pain in the cervical or thoracic areas, one of the most important issues is to distinguish between pain that occurs predominantly along the axis of the spine and radicular pain that is referred along the course of one or more spinal nerves.
Epidemiology
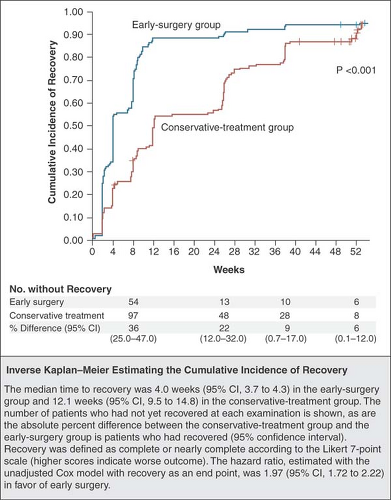
Low back pain is the second most common problem that leads patients to seek medical attention (2). The majority of episodes of acute low back pain with or without radicular pain will resolve without specific treatment. Overall, 60% to 70% of those who experience a first episode of acute low back pain will recover by 6 weeks, and 80% to 90% will do so by 12 weeks (Fig. 44-2) (3). For those with new onset of radicular pain due to an acute disc herniation, recovery with or without surgical discectomy occurs in nearly all individuals within the first 12 months (4); however, recovery is slow and uncertain. Fewer than 50% of those individuals disabled for longer than 6 months will return to work. The return-to-work rate for those absent from work for 2 years is near zero (5). There is a high lifetime recurrence rate, with the vast majority of those who have a single episode of back pain experiencing a second episode at some point during their lives (3). Both biologic and psychosocial factors lead some individuals to have a higher probability of developing chronic low back pain. Biologic factors predicting chronicity include longer duration of back pain, a past history of back pain, presence of leg pain, and obesity (3,6). Among the most predictive psychosocial factors are job dissatisfaction, depression, poor coping skills, and fear of reinjury. Chronic back pain is ubiquitous and a leading cause for medical intervention, disability, and long-term suffering among our patients.
Pathophysiology
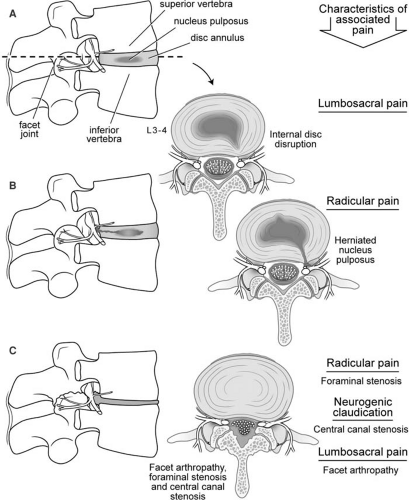
The basic functional unit of the spine is termed the functional spinal unit and is comprised of two adjacent vertebral bodies with two posterior facet joints, an intervertebral disc, and the surrounding ligamentous structures (Fig. 44-3). The intervertebral disc distributes the load evenly from one spinal segment to the next while allowing movement of the protective bony elements; it also serves to absorb energy (7). Mechanical stressors such as lifting, bending, twisting, or whole-body vibration have the potential to injure elements of the spine. With injury and aging, progressive degenerative changes appear in each element of the functional spinal unit, along with the onset of characteristic symptoms associated with specific degenerative changes (Fig. 44-3). The earliest change in the lumbar facet joints is synovitis, which progresses to degradation of the articular surfaces of the facet joints, capsular laxity and subluxation, and finally enlargement of the articular processes (facet hypertrophy). Progressive degeneration also occurs within the intervertebral discs, starting with the loss of hydration of the nucleus pulposus, followed by the appearance of circumferential or radial tears within the annulus fibrosis (internal disc disruption). Lumbosacral pain can arise from the degenerating facet joints or the annulus fibrosis, a structure that is richly innervated in the outer one-third of its circumference (8). With this internal disruption of the intervertebral disc, a localized protrusion of the disc called a disc herniation can appear (herniated nucleus pulposus or HNP). When disc material reaches the epidural space adjacent to the spinal nerve, this incites an intense inflammatory reaction (9). Those with HNP typically present with acute radicular pain. Hypertrophy of the facet joints and calcification of the ligamentous structures within the spinal canal can lead to a critical reduction in the dimensions of the intervertebral foramina and/or the central spinal canal (spinal stenosis), with the onset of radicular pain or neurogenic claudication.
An increasing number of patients present with prior lumbar surgery and either recurrent or persistent low back pain. This group, often termed failed back surgery syndrome, require special attention (10). All patients who have pain after prior lumbar surgery are not the same: Understanding what type of surgery was done, why the surgery was done, and the initial results of the surgery, as well as the time course and characteristics of any changes in the pattern and severity of symptoms is essential. Recurrent pain or progressive symptoms signal the need for further diagnostic evaluation in efforts to determine if a new and treatable cause for the pain can be identified.
Approach to Evaluation and Treatment
In the initial approach to evaluating any patient with low back pain, several key principles apply (Table 44-1). Features in the history that point to the need for prompt and detailed investigation include patients with new-onset or worsening back pain in the face of any history of trauma, infection, or previous cancer (Table 44-2). Those with progressive neurologic deficits (typically worsening numbness or weakness) or the appearance of bowel or bladder dysfunction also warrant immediate imaging to rule out a compressive lesion.
Because the lines of treatment available follow fairly discrete paths, it is reasonable to divide patients into four discrete categories, then proceed with diagnosis and treatment accordingly. First, determine if the pain is primarily along the axis of the cervical or lumbosacral spine or primarily radicular; then, establish the duration of the pain. Acute pain is often defined as pain that has been present less than 3 months, and chronic pain as pain that has been present for at least 3 months (1).
Acute Radicular Pain
A herniated intervertebral disc typically causes acute radicular pain, with or without radiculopathy (signs of dysfunction including numbness, weakness, or loss of deep tendon reflexes referable to a specific spinal nerve). In the elderly or those with extensive spondylosis, it is not uncommon to have the onset of acute radicular symptoms caused by narrowing of one or more intervertebral foramina. Initial treatment is symptomatic, and the symptoms will resolve without specific treatment in the majority of patients.
Chronic Radicular Pain
Persistent arm or leg pain in the distribution of a spinal nerve is not uncommon in those who have had a disc herniation, with or without subsequent surgery. In those with persistent pain, a search for a reversible cause for ongoing nerve root compression is warranted. In many individuals, extensive scarring surrounding the nerve root at the operative site can be seen on magnetic resonance imaging (MRI) (11), and electrodiagnostic studies will suggest typical patterns suggestive of chronic radiculopathy. This group is similar in characteristic to those who suffer from other chronic nerve injuries and have ongoing pain due to abnormal function of the nervous system, termed neuropathic pain. Like those with painful diabetic neuropathy and postherpetic neuralgia, this group is best approached initially with pharmacologic treatment for neuropathic pain.
Acute Cervical or Lumbosacral Pain
Most patients presenting with acute-onset axial cervical or lumbosacral pain without radicular symptoms will have no obvious signs of abnormality on physical examination, and imaging is unlikely to be of significant benefit in the acute setting. Traumatic sprain of the muscles and ligaments of the spine or the zygapophyseal joints, as well as early internal disc disruption, are all significant causes of acute cervical or lumbosacral pain. Like those with acute radicular pain, this group is best managed symptomatically with mild oral analgesics with or without muscle relaxants and prompt return to full activity.
Chronic Lumbosacral Pain
Perhaps the most challenging is the patient with persistent axial cervical or lumbosacral pain, particularly those with significant and ongoing disability. Chronic cervical and lumbosacral pain has many causes, and there is no means to identify the “pain generator” in any given individual with absolute certainty. The structures that have been most commonly implicated in causing chronic cervical or lumbosacral pain include the sacroiliac joint, the lumbar facets, and the intervertebral discs. The incidence of symptomatic conditions in these areas in patients presenting with chronic low back pain has been estimated to be 39% for internal disc disruption (range, 29%–49%), 15% for facet joint pain (10%–20%), and 15% for sacroiliac joint pain (7%–23%) (12). The gold standard for diagnosing sacroiliac and facet joint pain has been injection of local anesthetic
into the joint (13). However, the use of uncontrolled local anesthetic blocks for diagnostic purposes is plagued by a frequent placebo response and other factors (see Chapter 36). For those attaining significant pain relief with diagnostic blocks, radiofrequency treatment offers a simple, minimally invasive treatment that is modestly effective for treating facet-related pain. Finally, pain arising from degenerating intervertebral discs is also a common source of chronic axial neck or back pain. Diagnostic provocative discography has been used to identify symptomatic discs prior to intradiscal treatment with emerging therapies such as intradiscal electrothermal therapy (IDET) or surgical fusion; however, again, interpretation of response to provocative discography is not as straightforward as often claimed (see Chapter 38).
into the joint (13). However, the use of uncontrolled local anesthetic blocks for diagnostic purposes is plagued by a frequent placebo response and other factors (see Chapter 36). For those attaining significant pain relief with diagnostic blocks, radiofrequency treatment offers a simple, minimally invasive treatment that is modestly effective for treating facet-related pain. Finally, pain arising from degenerating intervertebral discs is also a common source of chronic axial neck or back pain. Diagnostic provocative discography has been used to identify symptomatic discs prior to intradiscal treatment with emerging therapies such as intradiscal electrothermal therapy (IDET) or surgical fusion; however, again, interpretation of response to provocative discography is not as straightforward as often claimed (see Chapter 38).
Medical Therapies
Neuropathic Pain Medications
Treatment of neuropathic pain in the form of chronic lumbar radicular pain is extrapolated from randomized trials examining the treatment of the more common forms of neuropathic pain, diabetic neuropathy and postherpetic neuralgia. Discrete characteristics suggest neuropathic pain and, in the presence of these abnormal sensations (Table 44-3), beginning treatment with oral neuropathic pain medications is warranted, even in the absence of an identifiable neurologic deficit. Anticonvulsants and antidepressants have shown significant efficacy in treating neuropathic pain. Tricyclic antidepressants (e.g., amitriptyline, nortriptyline, desipramine) and certain other antidepressants (i.e., bupropion, venlafaxine, duloxetine) are effective in the treatment of neuropathic pain (14). First-generation antiepileptic drugs (i.e., carbamazepine, phenytoin) and second-generation antiepileptic drugs (e.g., gabapentin, pregabalin) are also effective in the treatment of neuropathic pain (14).
Chronic Opioid Therapy
The use of chronic opioid therapy for the long-term treatment of noncancer pain remains a topic of significant controversy (15,16,17). Advocates point toward the significant long-term efficacy and improvement in function in patients with chronic painful conditions, including chronic low back pain. Opponents point to the difficulties with using these drugs over
the long-term (18). Although aberrant drug-related behavior (e.g., losing prescriptions, escalating drug dose beyond the prescriber’s recommendations) are relatively common in those receiving the medications for the treatment of chronic pain (19), overt addiction is uncommon (20). However, treating acute pain in the opioid-tolerant patient is a difficult proposition (21,22), and there is emerging recognition that chronic opioid use can actually worsen pain in the form of opioid-induced hyperalgesia (23). The current prevailing opinion seems to be that the use of modest doses of opioids can help to reduce pain and improve function in a select group of patients with chronic noncancer pain (24). However, the selection process is empiric, and there is no standard approach to choosing those who will do well.
the long-term (18). Although aberrant drug-related behavior (e.g., losing prescriptions, escalating drug dose beyond the prescriber’s recommendations) are relatively common in those receiving the medications for the treatment of chronic pain (19), overt addiction is uncommon (20). However, treating acute pain in the opioid-tolerant patient is a difficult proposition (21,22), and there is emerging recognition that chronic opioid use can actually worsen pain in the form of opioid-induced hyperalgesia (23). The current prevailing opinion seems to be that the use of modest doses of opioids can help to reduce pain and improve function in a select group of patients with chronic noncancer pain (24). However, the selection process is empiric, and there is no standard approach to choosing those who will do well.
When opting to treat a patient with long-term opioids, a wide array of opioid analgesics is available to choose from. The traditional paradigm for treating pain with opioids arose from the treatment of cancer pain (25). Using this approach, those with significant ongoing pain are given a long-acting opioid to provide continuous pain relief without the fluctuations in pain control associated with short-acting opioids. In addition, a small, intermittent dose of a short-acting agent is made available to treat pain that occurs with activity and “breaks through” the control provided by the long-acting agent alone. Nearly every available opioid has been used successfully in treating chronic low back pain, including short-acting agents alone or in combination formulations with ibuprofen or acetaminophen (e.g., hydrocodone, oxycodone) and long-acting agents (e.g., methadone, transdermal fentanyl, controlled-release oxycodone). Recently, a new type of opioid, termed ultra-fast onset, has emerged (e.g., oral transmucosal fentanyl citrate, fentanyl buccal tablet) for the rapid treatment of break-through pain (26). Like the patient selection process, choosing the best agent and the appropriate dose of opioid remains empiric. The choice between using short- or long-acting agents alone or in combination is best tailored to each patient and their individual pattern of pain. Ample evidence suggests that opioids significantly reduce pain during the first several months after initiating treatment, but studies of long-term treatment are just starting to appear (27).
Table 44-1 Key principles in the evaluation of patients with low back pain | |
|---|---|
|
Physical Therapy
Physical therapy, generally consisting of stretching, strengthening, and aerobic exercise in conjunction with patient education, is widely used and has shown benefit in treating patients with low back pain persisting beyond 6 weeks (28). Following acute lumbar strain with or without radicular pain, exercise therapy is no more effective than other conservative treatments, including no intervention (29). Even brief patient education through one-on-one, group, or video instruction can lead to significantly less disability and less worry about reinjury (30). Physical therapy can include the use of various modalities, including heat, ultrasound, and transcutaneous electrical stimulation (TENS); these modalities may provide short-term symptomatic relief, but evidence that they alter the long-term course of acute or chronic low back pain is lacking (29,31).
Table 44-2 Red flag for potentially serious conditions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Behavioral Therapy
Persistent pain is recognized as a problem that often has psychological and social/occupational dimensions, as well as biologic or physical components. Two main types of behavioral therapy have been used for back pain, operant conditioning and cognitive therapy. Operant conditioning aims to eliminate maladaptive pain behaviors. Cognitive therapy addresses how patients cope with their pain: what they actually do as a result of their pain and how their thoughts and feelings influence their behavior. Behavioral therapy significantly reduces pain intensity and behavioral outcomes (e.g., pain behavior, cognitive errors, perceived or observed levels of tension, anxiety, depression) compared with no treatment (32).
Multidisciplinary Pain Treatment Programs
The typical multidisciplinary treatment program includes a medical manager, usually a physician, who oversees all aspects of the treatment program, working together with other health care professionals who deliver behavioral therapy and physical therapy. In recent years, declining reimbursement has forced many inpatient programs to evolve to outpatient day treatment programs. Intensive (>100 hours of therapy) multidisciplinary biopsychosocial rehabilitation with functional
restoration significantly reduces pain and improves function compared with inpatient or outpatient non-multidisciplinary treatments or usual care; some improvements in physical function are sustained over long-term follow-up (33). Multidisciplinary pain treatment programs remain a viable and important treatment option for those with chronic pain accompanied by significant impairment in function.
restoration significantly reduces pain and improves function compared with inpatient or outpatient non-multidisciplinary treatments or usual care; some improvements in physical function are sustained over long-term follow-up (33). Multidisciplinary pain treatment programs remain a viable and important treatment option for those with chronic pain accompanied by significant impairment in function.
Table 44-3 The abnormal sensations of neuropathic pain | |
|---|---|
|
Other Therapies
Acupuncture
Determining the efficacy of acupuncture has been hindered by the lack of well-controlled trials (34). A recent meta-analysis analyzed 33 randomized clinical trials (RCTs) comparing acupuncture with sham intervention for the treatment of back pain (35). The investigators found that acupuncture effectively relieves chronic low back pain, but the data regarding the effectiveness of acupuncture for treating acute low back pain remains inconclusive. The effectiveness of acupuncture when compared to other available treatments has not been studied; the frequency and duration of treatment required to produce ongoing pain reduction also remain in question.
Spinal Manipulation
No universally accepted definition of spinal manipulation exists. In general terms, spinal manipulation involves the use of hands applied to the patient to deliver a forceful load to specific body tissues, typically with the intent of reducing pain and/or improving range of movement (36). Postulated mechanisms include increase of joint movement, changes in joint kinematics, increase of pain threshold, increase of muscle strength, and release of endogenous analgesic peptides (37). The available data are conflicting, but on balance show that spinal manipulation brings about a more rapid recovery in some patients if applied within 3 weeks of onset of acute low back pain (38). The outcomes regarding treatment of chronic low back pain are less clear, and the conclusions of systematic reviews are at odds. One recent systematic review concluded that spinal manipulation for chronic low back pain has an effect similar to an efficacious prescription of nonsteroidal anti-inflammatory drug; it is effective in the short-term when compared to placebo and general practitioner care, and in the long-term when compared to physical therapy (38). In contrast, another group systematically reviewed a series of 16 different systematic reviews regarding spinal manipulation and concluded that spinal manipulation was not effective for treating any condition, including low back pain (36). Nonetheless, they pointed to the paucity of data and called for additional clinical trials.
The Role for Neural Blockade: Interventional Pain Therapy
Interventional pain therapy refers to a group of targeted treatments used for specific spine disorders; many of these treatments evolved through modification of traditional techniques used for neural blockade for surgical anesthesia. These interventions range from epidural injection of steroids to percutaneous intradiscal techniques. Some of these interventional pain therapies have undergone extensive validation through RCTs, whereas others have progressed into widespread use without critical evaluation. When these treatment techniques are applied logically to the disorders that they have the most likelihood to benefit, they are a good addition to the armamentarium used to treat low back pain; when used haphazardly, they are unlikely to help patients and may be associated with significant risk of harm.
Despite the paucity of scientific evidence to guide pain practitioners, particularly evidence to support the use of many interventional modalities, a number of techniques appear to have efficacy based on limited observational data; these have been adopted into widespread use. Practitioners are left to choose among many available modalities, often with only anecdotal and personal experience to guide them, to treat a group of desperate patients with intractable pain who are willing to accept almost any intervention, even those which remain unproved. No single practice pattern exists that any pain specialist can point to as the correct way to treat patients with chronic pain. The best pain medicine practitioners strike a reasonable balance between interventional and noninterventional management. This practice pattern is sustainable, and those adopting a balanced style of practice will be able to adapt to evolving scientific evidence that appears in support of any particular pain treatment, regardless of its type. A balance between treatment modalities also allows practitioners to switch from one mode to another, or to incorporate multiple treatment approaches simultaneously.
Use of Image-guided Techniques in Pain Medicine
Little more than a decade ago, radiographic guidance was used infrequently by pain practitioners, being reserved for major procedures like neurolytic celiac plexus block. During the last several years, however, two forces have been at work. First, pain practitioners are now being called upon to serve as diagnosticians. Patients and referring practitioners expect pain physicians to have familiarity with imaging modalities and their usefulness in diagnosing pain conditions. At the same time, pain practitioners have come to realize the usefulness of radiographic guidance in achieving precise anatomic placement of needles and catheters. Although the evidence supporting the need for routine radiographic guidance is still evolving, the intuitive appeal of this more precise approach has caught firm hold, to the point where the majority of practitioners now perform at least a portion of their injections using fluoroscopic guidance (39). In some cases—as in patients with intractable pain associated with metastatic cancer—radiographic guidance has proven invaluable in the planning and implementation of therapy directed toward pain relief.
We have examined the distribution of injectate in a series of patients who received epidural steroid injections for radicular pain associated with a new herniated disc (40). We found that the injectate often spread to the side opposite the disc herniation. This is not at all surprising; if a disc herniation is present on one side, this might well obstruct the flow of fluid through the relatively confined epidural space. The fluid follows the path of least resistance, spreading preferentially to the contralateral, unaffected side and exiting the contralateral intervertebral foramina. This study and others (41,42) have challenged the conventional wisdom that suspending the steroid in a modest volume was sufficient to consistently produce spread of the injectate to the affected levels, regardless of where the solution was placed within the epidural space. Perhaps the blind loss-of-resistance technique is not the best way to deliver steroid to the site of inflammation.
Using radiographic guidance, bony structures can be visualized directly and in real time. The needle can be seen within the radiographic field, and simple geometry can be used to guide the needle directly from the skin’s surface to its destination. However, the field of pain medicine suffers from a lack of well-controlled studies to guide the choice of the most effective therapies. Indeed, many of the techniques described in this chapter lack clear evidence to support their efficacy. Even so, the techniques described here are in widespread clinical use. In the sections that follow, a clear summary of the current evidence available supporting the use of each technique has been given, but all too often these data are scant. With more consistent methodology, we can begin the much-needed work of assembling RCTs to determine which of these techniques are most useful in aiding those with intractable pain.
Specific Interventional Techniques
In the following sections, we provide an overview of the most common techniques used in interventional pain medicine. The section begins with a discussion of the anatomy relevant to image-guided interventions for treating chronic pain. Thereafter, we briefly describe the clinical utility of each technique and the technical aspects of conducting each intervention. We provide illustrations for the most common techniques, but detailed illustration of less common techniques is beyond the scope of this chapter. Many books have been published with detailed technical descriptions of these techniques, and we refer the interested reader to one of these texts (43).
Anatomy Relevant to Image-guided Intervention for Spinal Pain
The key to success in any interventional pain technique is a clear understanding of the normal anatomy. The procedures described in this text require understanding of the normal anatomy of the spine, including the epidural and subarachnoid spaces, the zygapophyseal joints, intervertebral discs and, most importantly, the spinal cord with its somatic and sympathetic components (see Chapter 9). In this section, we review the basic anatomy relevant to common interventions used in the treatment of chronic pain.
Anatomy of the Spine
The anatomy of the bony spine, individual vertebrae, meninges, spinal cord, spinal nerves, spinal vasculature, and cerebrospinal fluid (CSF) is described in detail in Chapter 9 (see Figs. 9-1,9-2,9-3,9-4,9-5,9-6,9-7,9-8,9-9,9-10,9-11,9-12,9-13,9-14,9-15,9-16,9-17,9-18,9-19,9-20,9-21,9-22,9-23,9-24,9-25,9-26,9-27,9-28,9-29,9-30,9-31,9-32,9-33,9-34,9-35,9-36,9-37,9-38,9-39,9-40).
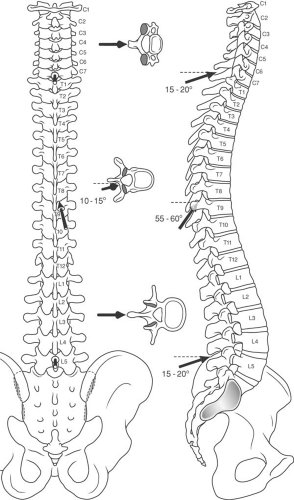
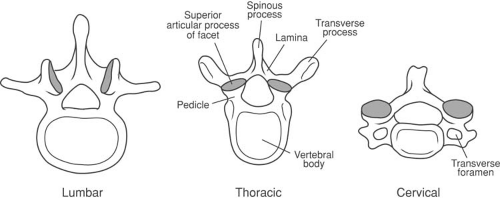
Normally, the vertebral canal is nearly triangular, surrounded by the bony components of the vertebrae. There are 33 vertebrae: 7 cervical, 12 thoracic, 5 lumbar, 5 fused elements that make up the sacrum, and 4 to 5 fused ossicles that form the coccyx (Fig. 44-4) (44). A typical vertebra consists of a vertebral body and two pedicles that extend posteriorly, surrounding the spinal canal and epidural space to join a pair of arched laminae (Fig. 44-5). The laminae fuse in the midline to form a dorsal projection called the spinous process. Near the junction of the pedicles and the laminae are found the lateral transverse processes, and the superior and the inferior articular processes (zygapophyses or facets). The pedicles and their articulating processes form the superior and inferior vertebral notches. In the articulated spine, these notches form the intervertebral foramina (44).
The zygapophyseal or “facet” joints are paired structures that lie posterolaterally on the bony vertebrae at the junction of the lamina and pedicle, medially, and on the base of the transverse process, laterally. The facet joints are true joints, with opposing cartilaginous surfaces and a true synovial lining, and they are subject to the same inflammatory and degenerative processes that affect other synovial joints throughout the body (44). Two opposing articular surfaces comprise each facet joint. The facet joint articular processes are named for the vertebra to which they belong. Thus, each vertebra has a superior articular process and an inferior articular process. This nomenclature can be confusing, as the superior articular process of a given vertebra actually forms the inferior portion of each facet joint.
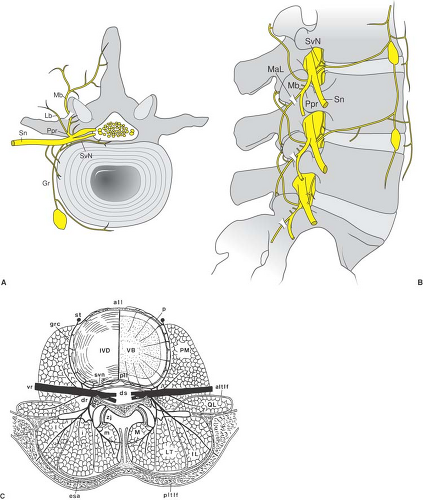
The intervertebral disc is comprised of glucosaminoglycans with a relatively fluid inner nucleus pulposus surrounded by a stiff, lamellar outer annulus fibrosis (44). With aging, the hydration of the intervertebral discs declines, leading to loss of disc height and fissure formation in the annulus fibrosis. These fissures begin centrally, near the border between the nucleus pulposus and the annulus fibrosis and can extend to the periphery of the disc space (Fig. 44-3B). This process of degradation is called internal disc disruption and is believed responsible for producing discogenic pain. The annulus contains neural elements from the sinuvertebral nerve, which is believed to be responsible for pain transmission (Figs. 44-6 and 44-7) (45). These same radial fissures within the annulus represent paths through which nuclear material can pass and extrude as a herniated nucleus pulposus. When this extruded material is adjacent
to an exiting spinal nerve root, it can lead to intense inflammation, nerve root compression, and radicular pain with or without radiculopathy (nerve root dysfunction in the form of numbness, weakness, and/or loss of deep tendon reflexes; Fig. 44-3B). The paired facet joints, along with the vertebral bodies and intervertebral discs, form the three weight-bearing support columns that distribute the axial load on the vertebral column while allowing for movement in various planes. The structure of the vertebrae varies from cephalad to caudad and should be thoroughly reviewed by the practitioner, especially when utilizing imaging (Figs. 44-4 and 44-5). Of importance when performing injections, the spinous processes of the cervical and lumbar regions approach the lamina in a nearly perpendicular fashion, which facilitates a midline approach when performing epidural or subarachnoid injections. The cervical facet joints are oriented nearly parallel to the axial plane, where the atlas (C1) articulates with the occiput, and become gradually more steeply angulated in a cephalad to caudad direction at lower cervical levels. The orientation of the cervical facet joints in a plane close to the axial plane allows for a great degree of rotation of the neck as well as flexion and extension.
to an exiting spinal nerve root, it can lead to intense inflammation, nerve root compression, and radicular pain with or without radiculopathy (nerve root dysfunction in the form of numbness, weakness, and/or loss of deep tendon reflexes; Fig. 44-3B). The paired facet joints, along with the vertebral bodies and intervertebral discs, form the three weight-bearing support columns that distribute the axial load on the vertebral column while allowing for movement in various planes. The structure of the vertebrae varies from cephalad to caudad and should be thoroughly reviewed by the practitioner, especially when utilizing imaging (Figs. 44-4 and 44-5). Of importance when performing injections, the spinous processes of the cervical and lumbar regions approach the lamina in a nearly perpendicular fashion, which facilitates a midline approach when performing epidural or subarachnoid injections. The cervical facet joints are oriented nearly parallel to the axial plane, where the atlas (C1) articulates with the occiput, and become gradually more steeply angulated in a cephalad to caudad direction at lower cervical levels. The orientation of the cervical facet joints in a plane close to the axial plane allows for a great degree of rotation of the neck as well as flexion and extension.
The mid thoracic (T5–T9) spinous processes are acutely angled caudad, making the midline approach to the epidural space more difficult than the paramedian approach (see Chapter 11). The thoracic facet joints are so steeply angulated that they approach the frontal plane, which makes intra-articular injection difficult or impossible. At mid thoracic levels, the inferior articular process of the vertebra forming the superior portion of each thoracic facet joint lies directly posterior to the superior articular process forming the inferior portion of each joint. This allows for some degree of flexion and extension, but limited rotation of the spinal column in the thorax.
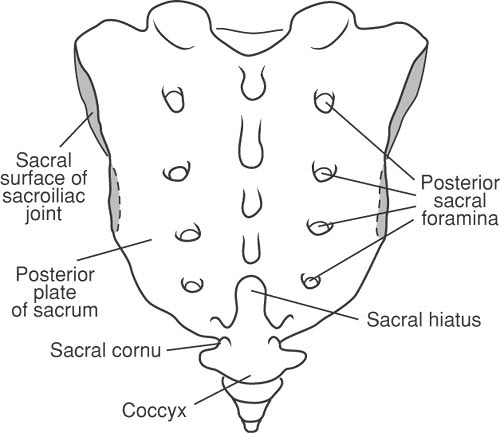
The spinous processes of the lumbar vertebrae approach the lamina in a nearly perpendicular fashion. The lumbar facet joints are angled with a somewhat oblique orientation, allowing for flexion, extension, and rotation that is greater than that in the thorax, but less than in the cervical region. The sacral hiatus is the area where the 5th sacral vertebra lacks both the laminae and the spinous process posteriorly (Fig. 44-8). The two sacral cornua lie on either side of the sacral hiatus and cephalad to the coccyx, and provide useful landmarks when performing an epidural from a caudal approach (see Chapter 11).
The midline distance from the ligamentum flavum to the dural sac varies considerably depending on the level of entry, increasing from 2 mm at C3–6 to 5 to 6 mm in the midlumbar region (46). The epidural space narrows posterior and laterally toward the intervertebral foramina. The anterior boundary of the epidural space is provided by the posterior longitudinal ligament covering the vertebral bodies and the intervertebral discs. Posteriorly, the epidural space is limited by the periosteum of the anterior surfaces of the laminae, the articulating facet processes (zygapophyses), and the ligamentum flavum. Laterally, the pedicles and the intervertebral foramina limit the epidural space (see Chapter 9, Figs. 9-5,9-6,9-7,9-8,9-9,9-10,9-11,9-12,9-13,9-14,9-15,9-16,9-17,9-18,9-19,9-20,9-21,9-22,9-23,9-24,9-25,9-26,9-27,9-28,9-29,9-30).
Knowledge of surface anatomy enhances safety when performing procedures under imaging and is of absolute necessity when imaging is not available. Important surface landmarks include the spinous process at C7 (vertebra prominens), which is the most prominent cervical spinous process palpable when the neck is flexed. The spinous process at T7 lies opposite the inferior angle of the scapula when the arm is at the side. A line joining the superior aspects of the iliac crests will pass through the spinous process of the fourth lumbar vertebrae. The spinal cord generally terminates at the L2 level, and the dural sac ends at S2, which corresponds to the level of the posterior superior iliac spines (see also Fig. 9-3). The tip of an equilateral triangle drawn between the posterior superior iliac spines and directed caudally overlies the sacral cornua and sacral hiatus.
Additionally, the articulated spine is supported by the anterior and posterior longitudinal ligaments, the supraspinous and interspinous ligaments, and ligamentum flavum (see Fig. 9-8) (46). Many of the pain management procedures discussed later will make reference to the ligamentum flavum. The ligamentum flavum or “yellow ligament” is a structure of variable thickness and completeness, composed of elastic fibers, that defines the posterolateral soft tissue boundaries of the epidural space. Because its leather-like consistency resists active expulsion of fluid from a syringe, loss of this resistance is valuable in signaling entry into the epidural space. The ligament’s structure is steeply arched and tent-like, so much so that the lateral reflection may be up to 1 cm deeper than at the midline. In the cervical and thoracic epidural spaces, the ligamentum flavum often does not fuse in the midline, which can become problematic during loss-of-resistance techniques. When the dense ligamentum flavum
is absent in the midline, it is possible to enter the epidural space without ever sensing significant resistance to injection. The ligamentum flavum is thickest at the lumbar and thoracic levels, and thinnest at the cervical level. Its thickness also diminishes at the cephalad aspect of each interlaminar space, and as the ligamentum flavum tapers off laterally (see Chapter 9, Figs. 9-5,9-6,9-7,9-8,9-9,9-10,9-11,9-12,9-13,9-14,9-15,9-16,9-17,9-18,9-19,9-20,9-21,9-22,9-23,9-24,9-25,9-26,9-27,9-28,9-29,9-30). In patients who have undergone previous spinal surgery, scarring of the posterior epidural space is common, such that the loss-of-resistance and the flow of injected solutions are less predictable.
is absent in the midline, it is possible to enter the epidural space without ever sensing significant resistance to injection. The ligamentum flavum is thickest at the lumbar and thoracic levels, and thinnest at the cervical level. Its thickness also diminishes at the cephalad aspect of each interlaminar space, and as the ligamentum flavum tapers off laterally (see Chapter 9, Figs. 9-5,9-6,9-7,9-8,9-9,9-10,9-11,9-12,9-13,9-14,9-15,9-16,9-17,9-18,9-19,9-20,9-21,9-22,9-23,9-24,9-25,9-26,9-27,9-28,9-29,9-30). In patients who have undergone previous spinal surgery, scarring of the posterior epidural space is common, such that the loss-of-resistance and the flow of injected solutions are less predictable.
The spinal cord is a cylindrical structure comprised of an external white matter and internal gray matter, protected by the bony vertebral column. White matter represents the myelinated ascending and descending tracts of the spinal cord, which conduct information to and from the brain. The gray matter contains axons, dendrites, and synaptic terminals arranged grossly in the shape butterfly wings. The spinal cord receives its vascular supply from arteries of the brain and from segmental spinal arteries of the subclavian artery, aorta, and iliac arteries (44). The posterior spinal cord receives its blood supply from a paired system of arteries arising from the posterior inferior cerebellar arteries. The anterior spinal artery (ASA) is a single, discontinuous vessel formed by the union of a terminal branch from each vertebral artery that descends along the anterior midline of the spinal cord. In all regions of the cord, the ASA provides the nutrition to approximately 75% of the cord tissue, including all of the gray matter (46), which makes this territory most vulnerable to ischemia (see Chapter 9).
The spinal nerve at each level traverses the intervertebral foramen and divides into anterior and posterior primary rami. The anterior ramus contains the majority of sensory and motor fibers at each vertebral level. Of importance, a small branch of the anterior ramus, the sinuvertebral nerve, provides neural branches to the posterior outer layers of the annulus of the disc (see Chapter 9, Fig. 9-39) (45). The posterior primary ramus, in turn, divides into a lateral branch that provides innervation to the paraspinous musculature and a small, variable sensory distribution to the skin overlying the spinous processes, whereas the medial branch courses over the base of the transverse process, where it joins with the superior articular process of the
facet joint and courses along the articular process to supply sensation to the joint. Each facet joint receives sensory innervation from the medial branch nerve at the same vertebral level, as well as from a descending branch from the vertebral level above; thus, two medial branch nerves must be blocked to anesthetize each facet joint (e.g., medial branch blocks at the base of the L4 and L5 transverse processes are needed to anesthetize the L4–L5 facet joint). The specific course of the medial branch nerves and cannula position for radiofrequency treatment at specific spinal levels is discussed in the sections that follow.
facet joint and courses along the articular process to supply sensation to the joint. Each facet joint receives sensory innervation from the medial branch nerve at the same vertebral level, as well as from a descending branch from the vertebral level above; thus, two medial branch nerves must be blocked to anesthetize each facet joint (e.g., medial branch blocks at the base of the L4 and L5 transverse processes are needed to anesthetize the L4–L5 facet joint). The specific course of the medial branch nerves and cannula position for radiofrequency treatment at specific spinal levels is discussed in the sections that follow.
Epidural Injection of Steroids: Interlaminar and Transforaminal Approaches
Numerous randomized trials have examined the efficacy of epidural injection of steroids for treating acute radicular pain. The rationale behind injecting glucocorticoid into the epidural space is to combat the inflammatory response associated with acute disc herniation (47). In acute radicular pain associated with herniated nucleolus pulposus, the evidence in systematic reviews (47), as well as more recent RCTs (48,49), demonstrates that epidural steroids reduce the severity and duration of pain between 3 and 6 weeks after onset. There do not appear to be any long-term reductions in pain or disability that stem from long-term epidural steroid use (47,50). Use of this therapy for lumbosacral pain without radicular symptoms has never been proven to be of benefit.
The theoretical background supporting the use of epidural steroids is based on the existence of inflammation as the basic pathophysiologic process. Nerve root edema has been demonstrated surgically and with computed tomography (CT) in patients with herniated discs (51). Inflammation of nerves in the presence of a herniated disc has further been confirmed during surgery (52), myelography (53), and histologic examinations (54,55). More recently, phospholipase A2 (PLA2), the rate-limiting enzyme in the conversion of arachidonic acid to prostaglandins and leukotrienes, has been found in high levels in patients undergoing discectomy for herniated disc (56). Other inflammatory mediators such as prostaglandins have also been shown to produce hyperalgesia (57). Clinically, improvement of pain has been shown to coincide with resolution or decrease in nerve root edema, despite a persistent herniated disc (45).
In laboratory animals, PLA2 has been implicated as a primary inflammatory mediator, and its administration in lumbar nerve roots produces motor weakness and decreased mechanical withdrawal thresholds (58). Histologic examination shows reversible demyelination, vacuolar degeneration in the nerve roots, and unclear axonal margins (54). In another animal model of radiculopathy, Hayashi et al. (59) ligated the left L4 and L5 nerve roots while surgically placing an epidural catheter with the tip between the ligated nerve roots. All rats demonstrated reversible motor weakness, which completely resolved in 4 weeks, and all of the animals exhibited thermal hyperalgesia. The rats were separated into groups receiving either epidural betamethasone alone, normal saline, or epidural betamethasone plus bupivacaine. All treatment groups demonstrated a transient reduction in thermal hyperalgesia, but both betamethasone groups showed prolonged benefit lasting 3 to 4 weeks.
Steroids decrease inflammation by inducing the biosynthesis of a PLA2 inhibitor and preventing prostaglandin generation (60,61). Steroids have also been shown to suppress ongoing discharge in chronic neuromas and prevent the development of ectopic neural discharges from experimental neuromas, which has been attributed to direct membrane action (62). Steroids may also block nociceptive input. Local application of methylprednisolone was found to reversibly block transmission in the unmyelinated C-fibers, but not in Aβ-fibers (63).
Patient Selection
Recent reviews of epidural steroid injection have yielded contradictory results, although considerable overlap occurred between the trials included in these reviews (64,65). Koes et al. (64) reviewed 12 RCTs on the efficacy of epidural steroid injections for low back pain and sciatica. One-half of the trials reported positive outcomes of epidural steroid injections, and the other half reported negative outcomes. There were significant flaws in the design of most studies included in this analysis, although there appeared to be no relationship between the methodologic quality of the trials and the reported outcomes. Koes et al. concluded that the efficacy of epidural injections has not yet been established.
Watts et al. (65) performed a meta-analysis of 11 placebo-controlled trials on the efficacy of epidural steroid injections in the treatment of sciatica (9 of the same trials were considered by Koes). The quality of the trials was generally good. A clinically relevant response to treatment was at least 75% improvement or reduction in pain. With respect to short-term pain relief (1–60 days), the pooled odds ratio (OR; based on 10 trials) was 2.61 (95% confidence interval [CI], 1.80–3.77); with respect to long-term pain relief (12 weeks to 1 year), the pooled OR (based on 5 trials) was 1.87 (95% CI, 1.31–2.68). Watts et al. concluded that epidural steroid injections are effective in the management of patients with sciatica.
In 1999, Nelemans et al. (66) performed another systematic review of RCTs on the efficacy of injection therapy in patients with low back pain. This review differed from the previous reviews because it was not restricted to epidural steroid injections, and it also considered epidural injections with anesthetics and other injection sites, such as facet joint and local injections. The Nelemans group’s review was restricted to RCTs that included patients with duration of low back pain of longer than 1 month; twenty-one RCTs met this criterion. Eleven studies compared injection therapy with placebo injections. The methodologic quality of many studies was low. There were
only three well-designed explanatory clinical trials: one on injections into the facet joints with a short-term OR of 0.89 (95% CI, 0.65–1.21) and a long-term OR of 0.90 (95% CI, 0.69–1.17); one on epidural injections with a short-term OR of 0.94 (95% CI, 0.76–1.15) and a long-term OR of 1.00 (95% CI, 0.71–1.41); and one on local injections with a long-term OR of 0.79 (95% CI, 0.65–0.96).
only three well-designed explanatory clinical trials: one on injections into the facet joints with a short-term OR of 0.89 (95% CI, 0.65–1.21) and a long-term OR of 0.90 (95% CI, 0.69–1.17); one on epidural injections with a short-term OR of 0.94 (95% CI, 0.76–1.15) and a long-term OR of 1.00 (95% CI, 0.71–1.41); and one on local injections with a long-term OR of 0.79 (95% CI, 0.65–0.96).
Within the six subcategories of explanatory studies, the pooled OR with 95% confidence intervals were: facet joint, short-term, 0.89 (0.65–1.21); facet joint, long-term, 0.90 (0.69–1.17); epidural, short-term, 0.93 (0.79–1.09); epidural, long-term, 0.92 (0.76–1.11); local, short-term, 0.80 (0.40–1.59); and local, long-term, 0.79 (0.65–0.96).
Nelemans et al. concluded that convincing evidence is lacking on the effects of injection therapies for low back pain, pointing toward the need for more well-designed explanatory trials in this field.
All three systematic reviews are now significantly outdated, and the Cochrane database review performed by Nelemans in 1999 was withdrawn in January 2005, citing the need for an update. It is worth noting that a 80% to 90% probability exists that patients with low back pain will recover within 3 months.
Newer Studies
Several more recent RCTs have been performed that point toward a limited role for epidural corticosteroid injections in reducing the duration of acute pain. The efficacy of epidural corticosteroid injection in the conservative management of sciatica was examined by Buchner et al. (67). Thirty-six patients with lumbar radicular pain due to herniated nucleolus pulposus were randomized to receive epidural steroid injections or no injection. At 2 weeks after injection, those receiving epidural steroid injections had superior improvement in straight-leg raise. There were no differences in pain reduction or functional status at 6 weeks or 6 months after injection. The authors concluded that epidural steroid injections can be recommended only in the acute phase for the conservative management of lumbosciatic pain.
Wilson-Macdonald et al. (68) conducted a prospective randomized trial of epidural steroid injection compared with intramuscular steroid injection in 93 patients with pain due to lumbar nerve root compression. All patients had been categorized as potential candidates for surgical nerve root decompression before treatment. A significant reduction of pain occurred early (35 days) in those having an epidural steroid injection, but no difference was noted in the longer-term effects (2-year follow-up). Eighteen percent of patients in the epidural group and 15% of those in the control group underwent operative decompression during the 2-year follow-up (p = NS).
In 2005, a large multicenter trial of epidural corticosteroid injections for sciatica appeared, the Wessex Epidural Steroid Trial (WEST) study (69). Two-hundred-twenty-eight patients with unilateral sciatica of 1 to 18 months’ duration were randomized to receive either three epidural steroid injections or three interligamentous injections over a 3-week period. At 3 weeks, those receiving epidural steroids demonstrated a significantly greater reduction in pain, but no difference between groups was seen from 6 to 52 weeks of follow-up. The authors concluded that epidural steroid injections afforded patients earlier relief of pain, but no long-term benefit in pain or need for surgery.
When earlier studies are reexamined, a similar early reduction in pain can be seen despite the lack of long-term benefit from epidural steroid injections. Indeed, the much-cited trial performed by Carrette et al. (70) examined the effectiveness of epidural steroid injections as compared with saline for the treatment of acute radicular pain due to disc herniation and concluded there were no long-term benefits of epidural steroid injection. In this RCT of 158 patients, although there were no demonstrable differences between epidural steroid and placebo treatment groups at 3 months following injection, there was significant earlier reduction in pain and improvement in sensory deficits (3 weeks after treatment) in those receiving epidural steroid injections.
The route of injection has also been the subject of much debate in recent years. The transforaminal approach to placing epidural steroids has been advocated as a means of delivering the steroid in high concentration directly to the site of inflammation near the spinal nerve within the lateral epidural space. A recent randomized trial (71) compared the efficacy of transforaminal versus interspinous corticosteroid injection in treating radicular pain in 31 patients. They demonstrated significantly better pain reduction in the transforaminal group at 30-day follow-up. A mailed questionnaire also revealed significantly better pain relief and daily activity levels at 6 months after injection. This small study warrants further validation in a larger controlled trial. We are still lacking studies that compare the transforaminal route to the interlaminar route.
Two additional recent reviews reinforce the conclusions of earlier publications regarding the use of epidural steroids. Young et al. (72) published a comprehensive review of epidural steroid injections for treating spinal disease and concluded that “lumbar epidural steroid injections are a reasonable nonsurgical option in select patients,” particularly for providing earlier resolution of pain in patients with lumbar radicular pain. In addition, The American Academy of Neurology’s Technology Assessment Subcommittee published a focused assessment of the use of epidural steroid injections to treat radicular lumbosacral pain (73). This group concluded that, “1) Epidural steroid injections may result in some improvement in radicular lumbosacral pain when assessed between 2 and 6 weeks following the injection, compared to control treatments (Level C, Class I–III evidence). The average magnitude of effect is small and generalizability of the observation is limited by the small number of studies, highly selected patient populations, few techniques and doses, and variable comparison treatments; 2) in general, epidural steroid injection for radicular lumbosacral pain does not impact average impairment of function, need for surgery, or provide long-term pain relief beyond 3 months.”
Collectively, the numerous studies and reviews that have been carried out examining the usefulness of epidural steroids for the treatment of acute radicular pain due to herniated nucleolus pulposus fail to show any long-term benefit in pain reduction nor do these injections obviate the need for surgical intervention. However, the bulk of the studies do demonstrate more rapid resolution of pain in those receiving epidural steroid injections. Thus, the role for epidural steroid injections in the conservative management of radicular pain is simply to facilitate earlier pain relief and return to full function.
Drug Selection for Epidural Steroid Injection
Most studies in the literature have used either a mixture of local anesthetics and steroid, saline with steroid, or steroid alone. The steroids most commonly used are either methylprednisolone acetate (Depo-Medrol) or triamcinolone diacetate (Aristocort). The doses of methylprednisolone most widely used in the literature vary from 80 mg to 120 mg, and the doses of triamcinolone most commonly used vary from 50 to 75 mg (31,74,75,76).
Methylprednisolone acetate has been approved for intramuscular, intrasynovial, soft-tissue, or intralesional injection. It is a glucocorticoid with an elimination half-life of 139 hours, with a range of 58 to 866 hours (77). Triamcinolone diacetate,
with an elimination half-life of 18 to 36 hours, possesses glucocorticoid properties while being essentially devoid of mineralocorticoid activity, thus causing little or no sodium retention. It has been approved for administration by intramuscular, intra-articular, or intrasynovial routes (78).
with an elimination half-life of 18 to 36 hours, possesses glucocorticoid properties while being essentially devoid of mineralocorticoid activity, thus causing little or no sodium retention. It has been approved for administration by intramuscular, intra-articular, or intrasynovial routes (78).
In a review of the literature, Kepes et al. found that methylprednisolone was the least irritating, most beneficial, and longest acting (79), whereas Delaney et al. prefer triamcinolone because of its excellent anti-inflammatory effect and low potential for sodium retention (80). No study has compared the effectiveness of triamcinolone and methylprednisolone, and they are probably equally effective. Both of these preparations contain polyethylene glycol, which has been found to impair nerve transmission in rabbit’s vagus nerve and cause degenerative lesions in rat sciatic nerves (81,82). Also, both preparations contain benzyl alcohol, which is potentially toxic when administered locally to neural tissue (83). Neither of these preparations should be used intrathecally.
Most practitioners dilute the steroid with local anesthetic or sterile saline, and the results are apparently comparable with either diluent (84). Some authors have recommended the use of local anesthetics “in the presence of muscle spasms” (85,86). However small the dose, the use of local anesthetic carries some risks, including hypotension, arrhythmias, and seizures from intravascular injections. Benzon (31) suggests that, since the results are comparable, the use of saline is probably sufficient. Some investigators have combined epidural methylprednisolone with morphine. In an initial study, Cohn et al. (87) showed encouraging results in postlaminectomy patients; however, a subsequent study was unable to confirm such beneficial effects (88).
Traditionally, the volume of diluent has depended on the site of injection, whether lumbar, caudal, or transforaminal. When using a caudal approach, 20 to 25 mL of a solution has been recommended to assure epidural spread cephalad to the desired level (89,94). When using a lumbar interlaminar approach, a volume of 5 to 10 mL has been recommended to reach those areas most commonly involved in the lumbar region (90). Other practitioners use smaller volumes (2–3 mL), especially when using the transforaminal approach. Some authors (91) have suggested that several nerve roots may also be inflamed in addition to those adjacent to the herniated or bulging disc, and recommend against the use of small volumes of diluent. Additionally, Wood et al. suggested diluting the steroid, after their study showed degenerative lesions in rat sciatic nerves attributed to the polyethylene glycol vehicle in the steroid preparation (82). The optimal volume of injectate and site of epidural placement remain unresolved.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree