Spinal, Epidural, and Caudal Anesthesia
David J. Kim
Jason M. Lewis
I. GENERAL CONSIDERATIONS
A. Preoperative assessment of the patient for regional anesthesia is similar to that for general anesthesia. The details of the procedure to be performed, including its anticipated length, patient position, and a complete review of any coexisting diseases, should be taken into account in determining the appropriateness of a regional technique.
B. The area where the block is to be administered should be examined for potential difficulties or pathology. Preexisting neurologic abnormalities should be well documented and the presence of kyphoscoliosis determined.
C. A history of abnormal bleeding and a review of the patient’s medications may indicate a need for additional coagulation studies.
D. Informed consent should be obtained including a detailed explanation of the planned procedure, with risks and benefits. Patients should be reassured that additional sedation and anesthesia can be given during the operation and that general anesthesia is an option if the block fails or the operation becomes more prolonged or extensive than originally thought. In some instances, it is planned from the onset to have a combination of regional and general anesthesia.
E. As with general anesthesia, patients should receive appropriate monitoring (see Chapter 10) and have an intravenous (IV) line in place. Oxygen, equipment for intubation and positive-pressure ventilation, and drugs to provide hemodynamic support must be available.
II. SEGMENTAL LEVEL REQUIRED FOR SURGERY
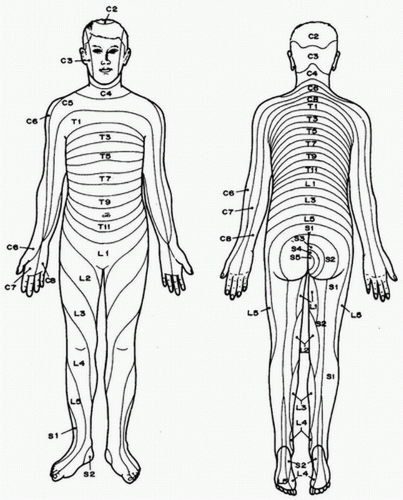
A. Knowledge of the sensory, motor, and autonomic distributions of spinal nerves will help the anesthetist determine the correct segmental level required for a particular operation and anticipate the potential physiologic effects of producing a block to that level. Figure 17.1 illustrates the dermatomal distribution of the spinal nerves.
B. Afferent autonomic nerves innervate visceral sensation and viscerosomatic reflexes at spinal segmental levels much higher than would be predicted from skin dermatomes.
C. Minimal suggested levels for common surgical procedures are listed in Table 17.1.
III. CONTRAINDICATIONS TO NEURAXIAL ANESTHESIA
A. Absolute
1. Patient refusal
2. Localized infection at skin puncture site
3. Significant coagulopathy
4. Increased intracranial pressure
B. Relative
1. Septicemia or bacteremia
2. Hypovolemia
3. Central nervous system disease
TABLE 17.1 Suggested Minimum Cutaneous Levels for Spinal Anesthesia | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
IV. SPINAL ANESTHESIA
Spinal anesthesia involves administering local anesthetic into the subarachnoid space.
A. Anatomy
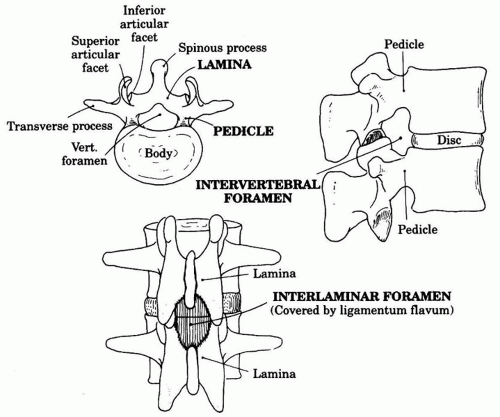
1. The spinal canal extends from the foramen magnum to the sacral hiatus. The boundaries of the bony canal are the vertebral body anteriorly, the pedicles laterally, and the spinous processes and laminae posteriorly (Fig. 17.2).
2. Three interlaminar ligaments bind the vertebral processes together:
a. Superficially, the supraspinous ligament connects the apices of the spinous processes.
b. The interspinous ligament connects the spinous processes on their horizontal surface.
c. The ligamentum flavum connects the caudal edge of the vertebrae above to the cephalad edge of the lamina below. This ligament is composed of elastic fibers and is usually recognized by its increased resistance to the passage of a needle.
3. The spinal cord extends the length of the vertebral canal during fetal life, ends at about L3 at birth, and is moved progressively to a cephalad position as the vertebral column grows to reach near the adult L1 level by 2 years of age. The conus medullaris, lumbar, sacral, and coccygeal nerve roots branch out distally to form the cauda equina. Spinal needles are placed in this area of the canal (below L2) because the mobility of the nerves reduces the danger of trauma from the needle.
4. The spinal cord is invested in three meninges:
a. The pia mater
b. The arachnoid, which lies between the pia and the dura mater
c. The dura mater, which is a tough fibrous sheath running longitudinally the length of the spinal cord and is tethered caudally at S2
5. The subarachnoid space lies between the pia mater and the arachnoid mater and extends from the attachment of the dura at S2 to the cerebral ventricles above. The space contains the spinal cord, nerves, cerebrospinal fluid (CSF), and blood vessels that supply the spinal cord.
6. CSF is a clear colorless fluid that fills the subarachnoid space. The total volume of CSF is 100 to 150 mL, whereas the volume in the spinal subarachnoid space is 25 to 35 mL. CSF is continuously formed at a rate of 450 mL/day by secretion or ultrafiltration of plasma from the choroid arterial plexuses located in the lateral, third, and fourth ventricles. CSF is reabsorbed into the bloodstream through the arachnoid villi and granulations that protrude through the dura to lie in contact with the endothelium of the cerebral venous sinuses.
B. Physiologic Changes
1. Neural Blockade. Differential blockade refers to the varying sensitivity of different types of nerve fibers to the effects of local anesthetics. Smaller C fibers conveying autonomic impulses are more easily blocked than the larger sensory and motor fibers. As a result, the level of autonomic blockade extends above the level of the sensory blockade by two to six segments. Similarly, fibers conveying sensation are more easily blocked than the larger motor fibers so that the sensory blockade will extend above the level of the motor blockade. Traditionally, it was believed that this differential blockade was solely due to difference in nerve fiber diameter. However, it is possible that the causes of this phenomenon are multifactorial.
2. Cardiovascular. Hypotension is directly proportional to the degree of sympathetic blockade produced. Sympathetic blockade results in dilatation of arteries and venous capacitance vessels, leading to decreased systemic vascular resistance and decreased venous return. If the block is below T4, increased baroreceptor activity produces an increase in activity to the cardiac sympathetic fibers and vasoconstriction of the upper extremities. Blockade above T4 interrupts cardiac sympathetic fibers, leading to bradycardia, decreased cardiac output, and a further decrease in blood pressure. These changes are more marked in patients who are hypovolemic, are elderly, or have obstruction to venous return (e.g., pregnancy). Risk factors for bradycardia after spinal anesthesia include baseline bradycardia, the American Society of Anesthesiologists physical status 1, use of β-blockers, age less than 50, prolonged PR interval, and sensory level above T6.
3. Respiratory. Low spinal anesthesia has no effect on ventilation. With ascending height of the block into the thoracic area, there is progressive ascending intercostal muscle paralysis. This has little effect on ventilation in the supine surgical patient with intact diaphragmatic function mediated by the phrenic nerve (C3 to C5), but patients may report a sensation of dyspnea due to decreased sensation of chest wall expansion. Conversely, ventilation in patients with poor respiratory reserve, such as the morbidly obese, may be profoundly impaired. Paralysis of both intercostal and abdominal muscles decreases the efficiency of coughing, which may be important in patients with chronic obstructive pulmonary disease. Usually, a spinal level of T4 does not result in impaired ventilation, but respiratory compromise may happen in patients with limited respiratory reserve or higher spinal levels.
4. Visceral Effects
a. Bladder. Sacral blockade (S2 to S4) results in an atonic bladder that can retain large volumes of urine. Blockade of sympathetic afferent and efferent innervation of the sphincter and detrusor muscle produces urinary retention.
b. Intestine. Sympathetic blockade (T5 to L1) produced by spinal anesthesia leads to contraction of the small and large intestines because of a predominance of parasympathetic tone.
5. Neuroendocrine. Peridural block to T5 inhibits part of the neural component of the stress response through its blockade of sympathetic afferents to the adrenal medulla and blockade of sympathetic and somatic pathways mediating pain. Other components of the stress response and central release of humoral factors are unaffected. Vagal afferent fibers from the upper abdominal viscera are not blocked and can stimulate release of hypothalamic and pituitary hormones, such as antidiuretic hormone and adrenocorticotropic hormone. Glucose tolerance and insulin release are normal.
6. Thermoregulation. Hypothermia may occur due to several mechanisms. The predominant cause is redistribution of the central heat to the periphery secondary to vasodilatation, which makes forced air warming particularly effective at raising the patient’s temperature. Core temperature may drop even though surface temperature is preserved and patients can feel warm despite a decrease in temperature. Thermoregulation is impaired given the loss of vasoconstriction to preserve heat below the level of sympathectomy. Shivering is common.
7. Central Nervous System Effects. Spinal anesthesia may have direct effects to suppress the consciousness, probably secondary to decreased afferent stimulation of the reticular activating system. During spinal or epidural anesthesia, doses of sedative agents may be decreased.
C. Technique
1. Spinal Needle. There are two main categories of spinal needles: those with a beveled tip that cut the dura (“cutting tip”) and those with a conical tip (“pencil-point”) with a lateral opening. The former includes the Quincke needle while the latter includes the Sprotte and Whitacre needles. Pencilpoint needles may reduce the incidence of postdural puncture headache (<1%) compared with traditional cutting-tip needles by splitting rather than cutting dural fibers during insertion. Twenty-four- and 25-gauge needles are easily bent and are often inserted through a 19-gauge introducer needle. The 22-gauge Quincke needle is more rigid and is easily directed when inserted. It can be useful in older patients in whom access may be more difficult and the incidence of postdural puncture headache is low.
2. Patient position. The lateral decubitus, prone, and sitting positions can be used for administration of spinal anesthesia.
a. In the lateral position, the patient is placed with the affected side up if a hypobaric or isobaric technique is to be used and with the affected side down if a hyperbaric technique is to be used. The spine is horizontal and parallel to the edge of the table. The knees are drawn up toward the chest, and the chin is flexed downward onto the chest to obtain maximal flexion of the spine.
b. The sitting position is useful for low spinal blocks required in certain gynecologic and urologic procedures and is commonly used in obese patients to assist in identification of the midline. It is often used in conjunction with hyperbaric anesthetics. The head and shoulders are flexed downward onto the trunk with the arms resting on a stand. An assistant should be available to stabilize the patient, and the patient should not be oversedated.
c. The prone position is used in conjunction with hypobaric or isobaric anesthetics for procedures on the rectum, perineum, and anus. A prone jackknife position can be used for administration of spinal anesthesia and the subsequent surgery.
3. Procedure
a. With all regional anesthetic procedures, the patient should be monitored with standard ASA monitors including EKG, blood pressure, and oxygen saturation.
b. The L2-L3, L3-L4, or L4-L5 interspaces are commonly used for spinal anesthesia. The L3 to L4 interspace or the spinous process of L4 is aligned with the upper borders of the superior iliac crests.
c. Disinfect a large area of skin with an appropriate antiseptic solution. Care must be taken to avoid contamination of the spinal kit with antiseptic solution as the solution is potentially neurotoxic.
d. Check the stylet for correct fit within the needle.
e. Raise a skin wheal with 1% lidocaine and a 25-gauge needle at the spinal puncture site.
f. Approaches
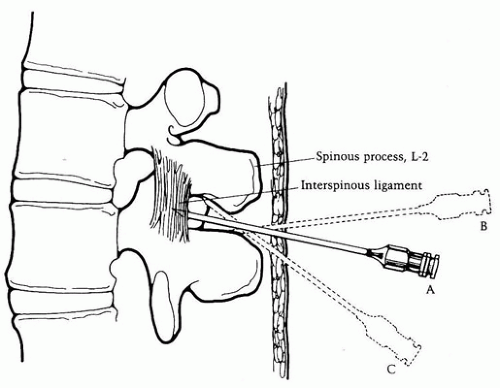
1. Midline. Place the spinal needle (or introducer) through the skin wheal and into the interspinous ligament. The needle should be in the same plane as the spinous processes and angulated slightly cephalad toward the interlaminar space (Fig. 17.3).
2. Paramedian. This approach is useful in patients who cannot adequately flex their backs because of pain or whose interspinous ligaments may be ossified. Place the spinal needle approximately 1 cm lateral and 1 cm caudad to the center of the selected interspace. Aim the needle medially and slightly cephalad, passing lateral to the supraspinous ligament. If the lamina is contacted, redirect the needle and walk the tip off the lamina in a medial and cephalad direction.
3. Needle Placement. Always keep the stylet in place when advancing the needle so that the needle’s lumen does not become plugged with tissue. If paresthesias occur during placement, immediately withdraw the needle. Allow the paresthesia to pass and reposition the needle before proceeding again. Advance the needle until increased resistance is felt as it passes through the ligamentum flavum. As the needle is advanced beyond this ligament, a sudden loss of resistance will occur as the needle “pops” through the dura.
4. Remove the stylet and confirm correct placement by noting free flow of CSF into the hub of the needle. Rotate the needle in 90-degree increments if necessary to confirm or reestablish good flow of CSF.
5. Administration of anesthetic. Connect the syringe containing the predetermined dose of local anesthetic to the needle. Gently aspirate CSF into the syringe, which produces birefringence within dextrose-containing solutions and confirms free flow. Inject the drug slowly. Repeat aspiration of CSF at the end of the injection confirms that the needle point is still within the subarachnoid space. Remove the needle and place the patient gently into the desired position.
g. Closely monitor (every 60 to 90 seconds) blood pressure, pulse, and respiratory function for 10 to 15 minutes. Determine the ascending anesthetic level by noting the response to a gentle pinprick or an alcohol swab. Stabilization of the local anesthetic level takes approximately 20 minutes.
h. Continuous spinal anesthesia allows small aliquots of drug to be injected repeatedly to produce the desired level of sensory blockade. With this technique, a high or rapid sympathetic block can be avoided (of particular concern in the compromised patient). A 20-gauge catheter is inserted through a 17-gauge epidural needle. The catheter is advanced 2 to 4 cm into the subarachnoid space. Stimulation of nerve roots during catheter insertion necessitates repositioning of the catheter. Neurotoxicity from hyperbaric glucosecontaining local anesthetic solutions injected through microbore spinal catheters (26 to 32 gauge) has been reported and may be due to the development of very high concentrations of local anesthetic around the nerves of the cauda equina. There are currently no such approved microbore catheters marketed in the United States.
i. Layered Spinal. This technique is frequently used in orthopedic surgery procedures at MGH to extend the duration of anesthesia by administering a dose of 0.5% bupivacaine through the spinal needle, waiting several minutes, and then adding additional drug incrementally.
D. Determinants of the Level of Spinal Blockade
1. Major
a. Baricity of Local Anesthetic Solution. Local anesthetic solutions can be described as hyperbaric, hypobaric, or isobaric in relation to the specific gravity of CSF (1.004 to 1.007 g/mL).
TABLE 17.2 Drugs and Dosages for Hyperbaric Spinal Anesthesia | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||
1. Hyperbaric solutions are typically prepared by mixing the drug with dextrose. They settle by gravity to the most dependent parts of the CSF column (Table 17.2).
2. Hypobaric solutions are prepared by mixing the drug with sterile water. They slowly rise to the highest part of the CSF column.
3. Isobaric solutions may have the advantage of a predictable spread through the CSF that is less dependent on patient position. Increasing the dose of an isobaric anesthetic has more of an effect on the duration of anesthesia than on the dermatomal spread. Patient positioning can be altered to limit or increase the spread of these mixtures.
b. Drug dose. The anesthetic level varies directly with the dose of the agent used.
c. Drug volume. The greater the volume of the injected drug, the further the drug will spread within the CSF. This is especially applicable to hyperbaric solutions.
d. Patient Position. This does not affect the spread of isobaric solutions.
2. Minor
a. Turbulence of CSF. Turbulence created within the CSF during or after the injection will increase the spread of the drug and the level obtained. Turbulence is created by rapid injection, barbotage (the repeated aspiration and reinjection of small amounts of CSF mixed with drug), coughing, and excessive patient movement.
b. CSF volume. Lumbosacral CSF volume is inversely correlated with the extent of the spread of local anesthetic. There are no good predictors of lumbosacral CSF volume, but weight has some correlation.
c. Increased intra-abdominal pressure. Pregnancy, obesity, ascites, and abdominal tumors increase pressure within the inferior vena cava. This pressure increases blood volume within the epidural venous plexus, concomitantly reducing the volume of CSF within the vertebral column, which permits greater spread of injected local anesthetic. In obese patients, this effect is potentiated by increased fat within the epidural space.
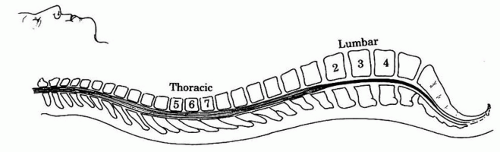
d. Spinal curvatures. Lumbar lordosis and thoracic kyphosis influence the spread of hyperbaric solutions. Drug injected above the L3 level while the patient is in the lateral position will spread cephalad and will be limited by the thoracic curvature at T4 (Fig. 17.4).
E. Determinants of the Duration of the Spinal Blockade
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree