CHAPTER 70
Spinal Cord Stimulation: Trialing Procedure
INTRODUCTION
This chapter deals with screening trials for the use of spinal cord stimulation (SCS) to treat otherwise intractable pain. The same general principles, however, apply to other types of neuromodulation for pain, such as peripheral, cortical, and deep brain stimulation, and stimulation for conditions other than pain.
SPECIFIC PURPOSES OF AN SCS SCREENING TRIAL
• To identify candidates for successful therapeutic chronic use of a stimulator and save the expense and morbidity of implantation in patients likely to be therapy failures.
• To predict the technical success of SCS (overlap of pain with a comfortable level of paresthesia and no motor reaction).
• To predict the clinical success of SCS (usually defined as ≥ 50% pain relief despite provocative activities, patient satisfaction with the treatment, and no increase in adjunct pain therapy).
• To determine if the patient can tolerate the sensation of paresthesia and can use the equipment successfully.
• To confirm patient compliance and validate patient reports by tracking usage.
• To reveal the electrode level and stimulation configuration that will optimize technical and clinical success.
• To gain information that will influence the choice of electrode(s) and the choice (rechargeable versus non-rechargeable) and location of the pulse generator in patients who proceed to implantation.
• To comply with the requirements of Medicare and many third-party payers.
APPROPRIATE PATIENT DIAGNOSES
• In many cases, the diagnosis underlying a chronic pain problem, and thus its prognosis and optimum treatment, is unclear.
• Experimental animal1 and human2 models indicate that SCS is more effective for neuropathic than for nociceptive pain.
• Animal models3 and clinical results point to the effectiveness of SCS for certain types of ischemic and visceral4 pain.
• We consider an SCS trial in a patient who has persistent or recurrent, otherwise intractable pain of the trunk and/or limbs. To the extent that a screening trial is a relatively low-risk, low-cost procedure, it is reasonable to offer screening liberally, ie, in cases in which the expected yield of success might be relatively low—in conjunction with critical interpretation of the results of the trial.
OFF-LABEL INDICATIONS OF SCS FOR PAIN
• Angina pectoris has been a popular indication for SCS in Europe,5 but is considered off-label by US manufacturers of SCS equipment. The yield of SCS screening trials in patients with angina pectoris is so high that the trial is often abbreviated or eliminated.
• Peripheral vascular disease causing limb pain, although clearly involving the areas covered by the US labeling language (pain of the trunk and limbs), is viewed conservatively as well. SCS can have a positive affect on blood flow, but this is not among the US label indications.
RELEVANT ANATOMY
• The effects of SCS vary according to which neural structures are stimulated.
• The location of the electrode (vertebral level6 and distance from the physiologic midline of the spinal cord7) dictates the anatomic area in which paresthesia can be elicited.
• The depth of cerebrospinal fluid (CSF), which conducts current from the electrode to the spinal cord, increases from the cervical to the mid-thoracic spine.
• Movement from the supine to the prone, sitting, or standing position increases the depth of CSF, which explains postural changes in stimulation.8
• Stimulation of large dorsal column afferents close to the pial surface of the spinal cord occurs at the lowest threshold with a dorsal midline electrode.
• Stimulation of dorsal roots can limit paresthesia coverage by causing reflex motor effects and discomfort. This is most likely to occur in the thoracic area where several millimeters of CSF and dura are interposed between the electrode and the pial surface.
PREOPERATIVE CONSIDERATIONS
• A reasonably current imaging study (MRI or CT myelography) of pertinent anatomy (typically the lumbar and thoracic spine) is essential for diagnosis and treatment planning.
• The patient’s use of any medication that can cause prolonged or excessive bleeding during surgery must be discontinued, typically 7 to 10 days before the procedure.
Percutaneous Trial Electrode Placement
• Placement of one percutaneous disposable trial electrode should be adequate for most patients, although such placement might be difficult to reproduce with an electrode for chronic use.
• Normal infection control precautions apply (sterile preparations, etc) and prophylactic IV antibiotic should be administered (depending on patient’s sensitivity) within an hour before beginning the procedure.
• Local anesthetic infiltration will control procedural pain and allow the patient to describe pain/paresthesia overlap.
• The patient is prone or seated with the target area neutral.
• Using a Tuohy needle, make a T12-L1 or L1-2 interlaminar puncture and advance at a shallow angle under anteroposterior (and, as necessary, lateral) fluoroscopy starting 1 to 2 segments below the target interlaminar space depending on the patient’s girth (Figure 70-1).
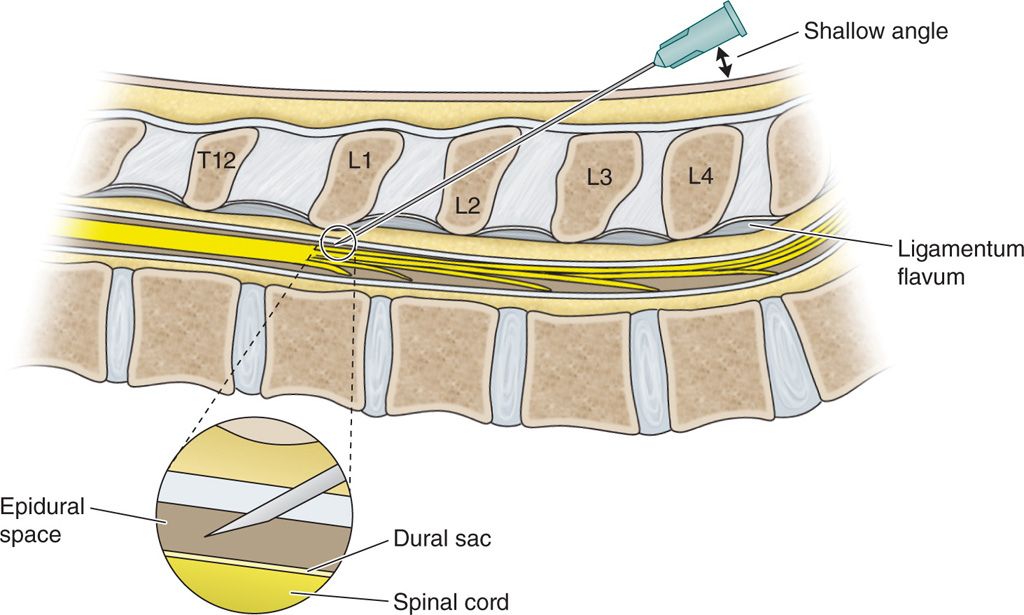
Figure 70-1. Using a Tuohy needle, make a T12-L1 or L1-2 interlaminar puncture.
• Increase the degree of spinal flexion as needed to gain additional interlaminar space.
• Confirm epidural space entry with loss of resistance to a Seldinger guidewire (injected air or saline can interfere with steering and with test stimulation).
• Stimulate using adjacent contacts (bipole, cathode cephalad) to identify the physiologic midline at each level as the electrode is advanced incrementally along the radiographic midline to the desired longitudinal location, repositioning as needed to achieve the requisite symmetry and pain/paresthesia overlap.
• If targeting the low back, the electrode must be close to the physiologic midline, ie, the bilateral/perceptual stimulation threshold ratio should be no more than 110% (Figure 70-2).
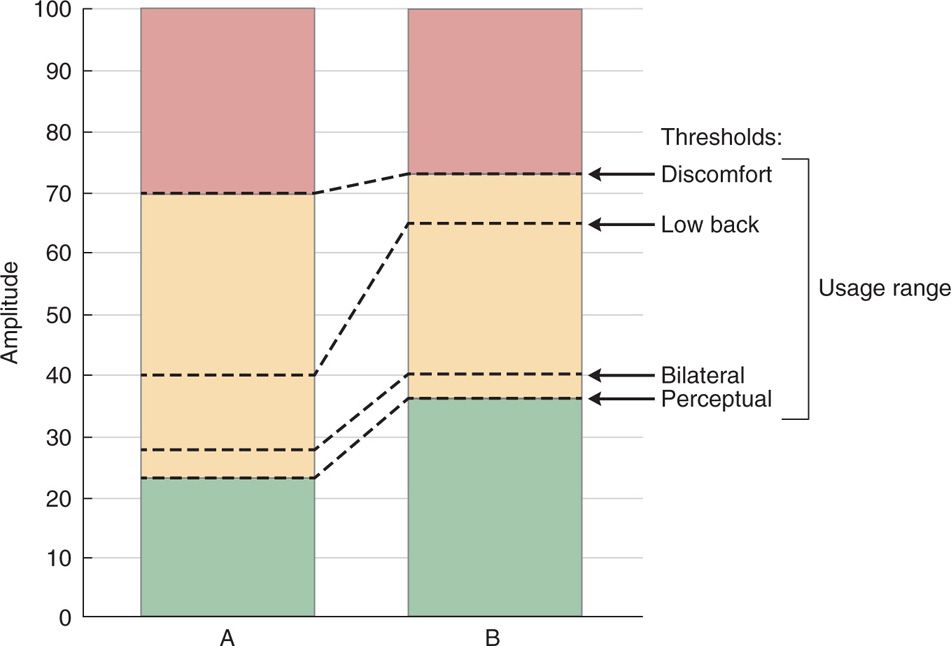
Figure 70-2. Clinically relevant stimulation falls within an amplitude that ranges from perception to discomfort, illustrated here for configurations A and B. The amplitudes at which bilateral effects are perceived and paresthesia overlaps low back pain can be scaled to this usage range. In this example, A and B are comparably symmetric, in that their ratio of bilateral to perceptual threshold is similar. In A, low back coverage occurs close to the perceptual threshold; in B, low back coverage occurs near the discomfort threshold.
• If a patient has leg pain predominant on one side and electrode placement is to favor one side, it should favor the side with the predominant pain.
• When the contacts are in a stable position along the radiographic midline, begin test stimulation to:
![]() Familiarize the naïve patient with the sensation of paresthesia and the test routines.
Familiarize the naïve patient with the sensation of paresthesia and the test routines.
![]() Orient the operator to the physiologic midline, which often differs from the radiographic midline.
Orient the operator to the physiologic midline, which often differs from the radiographic midline.
![]() Record areas of paresthesia coverage at multiple amplitudes: perceptual, bilateral, usage, and discomfort.
Record areas of paresthesia coverage at multiple amplitudes: perceptual, bilateral, usage, and discomfort.
![]() Map responses that might be useful clinically (even if the electrode is well below the anticipated optimal level, eg, to cover a secondary area of foot pain).
Map responses that might be useful clinically (even if the electrode is well below the anticipated optimal level, eg, to cover a secondary area of foot pain).
• Leaving the percutaneous electrode in place, withdraw the Tuohy needle, and suture the lead to skin.
• Place the trial connector on the side appropriate for placement of the pulse generator, eg, the side opposite the one the patient sleeps on.
• Occasionally, multiple electrode arrays will be inserted for a trial:
![]() At 2 different levels to cover pain at 2 different levels, eg, the low back and the foot.
At 2 different levels to cover pain at 2 different levels, eg, the low back and the foot.
![]() Side by side, eg, for bilateral foot pain (note that this configuration is inferior for low back pain, at least acutely).9
Side by side, eg, for bilateral foot pain (note that this configuration is inferior for low back pain, at least acutely).9
![]() In 3 columns to create transverse tripole stimulation, which is cumbersome with percutaneous electrodes because it requires 3 needle sticks or a specialized catheter and because of the difficulty in maintaining the relative position of the electrodes.
In 3 columns to create transverse tripole stimulation, which is cumbersome with percutaneous electrodes because it requires 3 needle sticks or a specialized catheter and because of the difficulty in maintaining the relative position of the electrodes.
• A percutaneous electrode designed for chronic therapy can be used for the trial and implanted with the expectation that it will remain in place if the trial is successful (as is common in Europe). This requires extra steps (incision, tunneling, anchoring) and incurs some disadvantages:
![]() Additional pain can confound trial interpretation.
Additional pain can confound trial interpretation.
![]() The increased risk of infection might cause the trial to be expedited inappropriately.
The increased risk of infection might cause the trial to be expedited inappropriately.
![]() With the most difficult step of implantation completed, a certain amount of momentum has been gained toward proceeding with generator implantation, and the trial might be interpreted less critically than it should.
With the most difficult step of implantation completed, a certain amount of momentum has been gained toward proceeding with generator implantation, and the trial might be interpreted less critically than it should.
• Note on high frequency and burst stimulation: Patients might not feel SCS paresthesia at very high frequencies, delivered continuously or in bursts; when the use of these waveforms is contemplated, conventional, lower frequencies can be used to guide electrode placement.
Plate/Paddle Trial Electrode Placement
• Plate or paddle electrodes should be reserved for trials in special circumstances:
![]() Percutaneous access is not feasible (eg, after spine surgery at the planned level of insertion).
Percutaneous access is not feasible (eg, after spine surgery at the planned level of insertion).
![]() To mitigate painful side effects (such as stimulation-evoked axial pain) likely mediated by small fibers in the ligamentum flavum.10
To mitigate painful side effects (such as stimulation-evoked axial pain) likely mediated by small fibers in the ligamentum flavum.10
• Plate or paddle electrodes used for trials are typically preserved for chronic use if the trial is successful.
• It is not necessary to use a paddle electrode to reduce migration; contemporary percutaneous electrode anchoring techniques have reduced the incidence of percutaneous electrode migration to nearly zero.11
• Normal infection control precautions apply (sterile preparations, etc), and prophylactic IV antibiotic should be administered (chosen subject to patient’s sensitivity) within an hour before beginning the procedure.
• Paddle electrodes can be inserted using a variety of anesthetic techniques:
![]() Infusion and/or intermittent boluses of IV sedation (eg, propofol) and local anesthetic minimize discomfort for the patient while allowing for verification of electrode location as well as intraoperative testing of neurological function.
Infusion and/or intermittent boluses of IV sedation (eg, propofol) and local anesthetic minimize discomfort for the patient while allowing for verification of electrode location as well as intraoperative testing of neurological function.
![]() Epidural, subarachnoid or intrathecal spinal anesthesia12 can provide local analgesia without inducing motor or sensory blockade, while still allowing stimulation paresthesia to be elicited to determine appropriate placement in most cases, albeit at higher amplitudes. Importantly, on the other hand, these types of anesthesia can block pain that signals injury, which can result in a complete motor block, making it impossible to evaluate motor function and possible spinal cord compression until well after completion of the procedure.
Epidural, subarachnoid or intrathecal spinal anesthesia12 can provide local analgesia without inducing motor or sensory blockade, while still allowing stimulation paresthesia to be elicited to determine appropriate placement in most cases, albeit at higher amplitudes. Importantly, on the other hand, these types of anesthesia can block pain that signals injury, which can result in a complete motor block, making it impossible to evaluate motor function and possible spinal cord compression until well after completion of the procedure.
![]() General anesthesia, using evoked potential monitoring to guide placement in the physiological midline,13 precludes patient feedback but allows for monitoring of somatosensory and motor evoked potentials, which can provide information regarding the integrity of spinal cord pathways relative to their preoperative status.
General anesthesia, using evoked potential monitoring to guide placement in the physiological midline,13 precludes patient feedback but allows for monitoring of somatosensory and motor evoked potentials, which can provide information regarding the integrity of spinal cord pathways relative to their preoperative status.
• With the patient prone or semilateral, use bolsters or a radiolucent Wilson frame to:
![]() Allow precise positioning of a C-arm fluoroscope, which is helpful for verification of anatomic midline placement.
Allow precise positioning of a C-arm fluoroscope, which is helpful for verification of anatomic midline placement.
![]() Avoid lateral curvature or rotation of the patient’s spine, which might compromise accurate midline placement.
Avoid lateral curvature or rotation of the patient’s spine, which might compromise accurate midline placement.
![]() Improve the surgeon’s orientation compared with the lateral position.
Improve the surgeon’s orientation compared with the lateral position.
• Confirm that skin rolls or hyperextended posture do not impede access to the implant site(s). Orient the C-arm to provide a true AP view, with the spinous processes positioned in the midline between the pedicles. Aligning the C-arm beam with the end plates ensures accurate localization.
• After sterile prep and draping, infiltrate the skin and paravertebral muscles with local anesthetic.
• Under fluoroscopy, center a 1- to 2-in incision on the implantation target area, which should be just caudal to the final position planned for the most caudal contact.
• Dissect the paravertebral muscles subperiosteally; place self-retaining retractors.
• Perform a mini laminectomy of sufficient breadth, and length, oriented to allow shallow-angle insertion of the electrode beneath the intact lamina(e) above. Keep the force vectors in the rostrocaudal dimension rather than down toward the dura and spinal cord.
• Some clinicians have described a minimally invasive paramedian approach using a tubular retractor system.14 Although the least invasive approach is desirable, the highest priority is safe and technically accurate electrode insertion, achieving a stable long-term placement.
• Perform physiological monitoring to verify electrode placement and maximize pain/paresthesia overlap. The most straightforward method is verbal reporting from a patient who is awake. Electrophysiological monitoring of motor and sensory evoked potentials might alert the surgeon to the potential of spinal cord injury before permanent injury can occur. Ultimately, the choice of physiological confirmation method depends on the experience and judgment of the surgeon.
• Anchor the lead wire to the supraspinous ligament using a sleeve/strain relief and (author’s preference) silicone elastomer adhesive.
• Place the trial connector on the side appropriate for placement of the pulse generator, eg, the side opposite the one the patient sleeps on.
POSTPROCEDURE CONSIDERATIONS
• Because patients seldom use SCS while in the prone position in which percutaneous electrodes are placed, promptly following electrode placement, program the temporary stimulator to optimize paresthesia coverage of pain with the patient sitting or supine.
• Before discharge, while monitoring the patient postoperatively for neurologic and other potential complications, continue programming and patient education as needed.
• Pay careful attention to postoperative pain medication use, which can confound interpretation of the trial.
• Each SCS manufacturer provides a temporary external generator. (Constant current as well as constant voltage generators are available, but we lack high quality evidence about their comparative advantages.)
• A 1-week trial is our usual practice. The trial can be extended to approximately 3 weeks (as in many European centers), however, or conducted in minutes while the patient is “on the table.”15
• In cases where an otherwise successful trial is accompanied by therapy-limiting side effects, such as unwanted stimulation, a repeat trial can be conducted with an insulated plate/paddle electrode (perhaps “on-table” during permanent implant).
• A patient who reports sufficient and satisfactory pain relief during a screening trial of reasonable duration with stable or reduced analgesic use and a concordant increase in activity should be offered an implanted system.
MONITORING OF POTENTIAL COMPLICATIONS
• Spinal cord or nerve injury (including epidural hematoma) requires emergency neurosurgical management, which can include decompression.
• Dural puncture responds to bed rest, hydration, caffeine, and (when necessary) an epidural blood patch.
• Infection (wound or skin breakdown) requires a culture specimen, removal of the system (in most cases), and administration of appropriate antibiotics.
• Electrode migration might be mitigated by reprogramming the contacts noninvasively; if this is not sufficient, the electrode(s) may be repositioned or replaced.
• Electromechanical failure requires revision.
CLINICAL PEARLS AND PITFALLS
• Our reliance on screening trials is presumptive. We even lack randomized controlled trial evidence, for example, on the impact of extending or eliminating screening trials.
• For patients with failed back surgery syndrome, we routinely insert the percutaneous trial electrode at T12-L1 or L1-L2.
![]() Because the tip of the conus is at L1-L2, a dorsal epidural electrode below this level will be delivering cauda equina stimulation rather than SCS. The rootlets of the cauda are mobile and difficult to recruit consistently.
Because the tip of the conus is at L1-L2, a dorsal epidural electrode below this level will be delivering cauda equina stimulation rather than SCS. The rootlets of the cauda are mobile and difficult to recruit consistently.
![]() Insertion into the relatively immobile thoracic spine makes the body of the lead less subject to stresses and strains than would be the case in mobile lumbar segments.
Insertion into the relatively immobile thoracic spine makes the body of the lead less subject to stresses and strains than would be the case in mobile lumbar segments.
![]() Rarely, the interlaminar spaces are too small at the recommended levels, and one must look lower.
Rarely, the interlaminar spaces are too small at the recommended levels, and one must look lower.
![]() Not uncommonly, the patient has had a laminectomy up to and including the upper lumbar levels, and this constrains the approach to be more cephalad.
Not uncommonly, the patient has had a laminectomy up to and including the upper lumbar levels, and this constrains the approach to be more cephalad.
![]() Even when the more caudal interlaminar spaces are available, they are less desirable because lumbar lordosis makes it more difficult to achieve the requisite shallow angle of insertion.
Even when the more caudal interlaminar spaces are available, they are less desirable because lumbar lordosis makes it more difficult to achieve the requisite shallow angle of insertion.
![]() Furthermore, if the patient has low back pain, introducing a needle at one of the lower lumbar interlaminar spaces is likely to cause acute pain in the same location as the patient’s usual pain, which might confound interpretation of the trial.
Furthermore, if the patient has low back pain, introducing a needle at one of the lower lumbar interlaminar spaces is likely to cause acute pain in the same location as the patient’s usual pain, which might confound interpretation of the trial.
• It is commonly the case that de novo placement of an electrode for chronic use offers the opportunity for the operator as well as the patient to improve upon results obtained with the temporary trial electrode.
![]() The previously naive patient has gained experience with the technical goals of the procedure, and the operator has gained experience with the individual patient.
The previously naive patient has gained experience with the technical goals of the procedure, and the operator has gained experience with the individual patient.
![]() The operator can choose a permanent electrode based on experience with the temporary.
The operator can choose a permanent electrode based on experience with the temporary.
![]() Thus, we prefer a strictly temporary percutaneous electrode for SCS screening trials when feasible—as is fortunately the case in most patients.
Thus, we prefer a strictly temporary percutaneous electrode for SCS screening trials when feasible—as is fortunately the case in most patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






