Specific Considerations with Cardiac Disease
Christina Anne Jelly
Olof Viktorsdottir
I. GENERAL CONSIDERATIONS
An estimated one in three of Americans has one or more types of cardiovascular disease (CVD). Mortality data show that one of every three deaths in the United States is secondary to CVD.
II. CORONARY ANATOMY
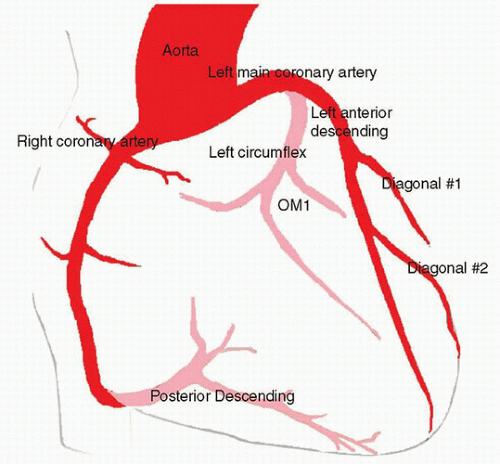
The coronary arteries perfuse the myocardium. The left and right coronary arteries originate from the coronary sinuses distal to the aortic valve. The left main coronary artery (LMCA) branches into the left anterior descending artery (LAD) and the left circumflex artery (LCX) to supply most of the left ventricle (LV), interventricular septum (IVS), and the left atrium (LA). The right coronary artery (RCA) supplies the right atrium (RA) and ventricle (RV). It also supplies portions of the IVS, including the sinoatrial (SA) and atrioventricular (AV) nodes (Fig. 2.1). In approximately 70% of the population, the posterior descending artery (PDA) is supplied by the RCA. This circulation is described as “right dominant.” The remainder of the population is either “left dominant,” with the PDA emerging from the LCX (10% of the population), or “codominant,” with the PDA receiving contributions from both the LCX and RCA (20% of population).
III. PREOPERATIVE CARDIOVASCULAR EVALUATION FOR NONCARDIAC SURGERY
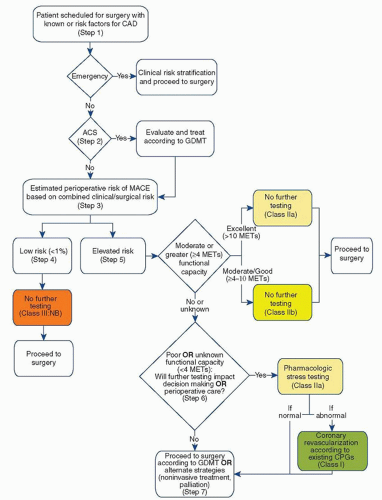
The American College of Cardiology and the American Heart Association (ACC/AHA) have developed joint guidelines for the preoperative cardiovascular evaluation of patients undergoing noncardiac surgery. The initial evaluation consists of the patient’s history, focused physical examination, and routine laboratory investigation. Based on the patient’s history, cardiac risk factors, functional status, and the nature of the surgical procedure, the ACC/AHA guidelines provide a stepwise approach for identifying patients who may benefit from further cardiovascular testing (Fig. 2.2).
A. Initial Screening
1. The need for emergency surgery preempts further cardiac workup. The ACC/AHA guidelines define an emergency procedure as one that must be started within 6 hours. As there is little to no time, the patient must proceed often with no or very limited clinical preoperative evaluation. Emergent surgery should proceed with appropriate patient monitoring and management strategies based on the patient’s clinical risk factors for CAD.
2. If the surgery is not emergent, determine whether the patient has an acute coronary syndrome (ACS). An ACS is unstable angina or a myocardial infarction (MI). The patient may present with chest pain, shortness of breath, diaphoresis, or nausea. The EKG may show ST segment depressions or elevations. If the patient has an ACS, the surgical procedure should be postponed and the patient should immediately undergo cardiac evaluation and guideline-directed medical therapy (GDMT).
If the patient does not have an ACS, then proceed with an assessment of the patient’s postoperative risk for a major adverse cardiac event (MACE).
If the patient does not have an ACS, then proceed with an assessment of the patient’s postoperative risk for a major adverse cardiac event (MACE).
B. Risk of MACE. The patient’s risk of MACE should be determined based on clinical characteristics and the surgical procedure. Clinical risk factors for MACE include a history of heart failure, coronary artery disease, cerebrovascular disease, diabetes, and chronic kidney disease. Validated risk prediction tools, such as the Revised Cardiac Risk Index (RCRI) and the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) Surgical Risk Calculator (riskcalculator.facs.org), can be used to help predict the risk of perioperative MACE.
1. If the patient has a low risk of MACE (<1%), then no further testing is needed and the patient may proceed to surgery.
2. If the patient has an elevated risk of MACE (≥1%), then the functional capacity should be determined. Functional capacity can be expressed as metabolic equivalents (METs). A single MET represents oxygen consumption at rest. If the patient has moderate or excellent functional capacity without cardiac symptoms, then the patient may proceed to surgery without further evaluation. Moderate or excellent functional capacity is defined as exercise capacity greater than or equal to 4 METs. Activities that correspond to moderate functional capacity include climbing two flights of stairs, walking on level ground at 4 mph, running a short distance, scrubbing floors, or playing a game of golf without a cart. The ability to participate in strenuous sports such as swimming, singles tennis, or football generally corresponds to excellent functional capacity.
3. If the patient has poor or unknown functional capacity, then further testing may be needed if the results will change a patient’s management. Poor functional capacity is defined as an exercise capacity less than 4 METs. Examples include the inability to walk more than two blocks on level ground without stopping due to symptoms and activity limited to eating, dressing, and walking indoors. Further testing may include exercise or pharmacologic stress testing. If abnormal, coronary angiography may be considered. The patient can then proceed to surgery with
GDMT. Testing may not be necessary if the results will not alter patient management.
GDMT. Testing may not be necessary if the results will not alter patient management.
C. Supplemental Cardiac Evaluation. Supplemental cardiac evaluation should be performed when indicated to measure functional capacity, identify the presence of cardiac dysfunction, and provide an estimate of the perioperative cardiac risk.
1. Preoperative resting 12-lead ECG should be considered for patients with known coronary heart disease, significant arrhythmia, peripheral arterial disease, cerebrovascular disease, or other structural heart disease, except for those undergoing low-risk surgery. Routine preoperative ECG is not indicated for asymptomatic patients undergoing low-risk surgery.
2. Rest echocardiography may be used to evaluate ventricular function in patients with history of heart failure or dyspnea of unknown origin. It is also useful to assess valvular pathology in patients with a history of valvular disease or a newly identified heart murmur.
3. Stress testing is recommended for patients with elevated risk of MACE and poor or unknown functional capacity if outcome will change management. Patients who have an elevated risk of MACE and moderate to good (4 to 10 METs) functional capacity may proceed to surgery without stress testing. Routine screening with stress testing is not useful for patients undergoing low-risk noncardiac surgery.
a. Exercise stress testing provides an objective measure of functional capacity. It is the preferred modality in patients who are capable of achieving adequate workloads. Sensitivity and specificity for multi-vessel CAD are 81% and 66%, respectively. Exercise stress tests are highly predictive when ST segment changes are characteristic of ischemia (>2 mm, sustained into recovery, and/or associated with hypotension). The risk of perioperative cardiac events is increased significantly in patients who have abnormal exercise ECGs at low workloads. Radionuclide imaging or echocardiography can be combined with exercise stress testing for patients whose baseline ECGs render interpretation invalid.
b. Pharmacologic stress testing can be conducted with either an agent that increases myocardial oxygen demand (dobutamine) or dilates coronary arteries (dipyridamole or adenosine). Pharmacologic stress tests are suitable for patients who are unable to exercise. Dobutamine stress testing is typically combined with echocardiography to detect wall motion abnormalities brought about by the increased myocardial workload. Dipyridamole or adenosine stress tests are typically combined with radionuclide imaging to detect areas of myocardium that are at risk. Pharmacologic vasodilation has the risk of a false-negative test in patients with multivessel CAD where all vessels are already maximally vasodilated. In both cases, perioperative cardiac risk is directly proportional to the extent of myocardium that is found to be at risk on imaging.
4. Cardiac catheterization is considered the “gold standard” for evaluating CAD. Information obtained includes coronary anatomy with visualization of direction and distribution of flow, hemodynamics, and overall function of the heart. Routine preoperative coronary angiography is not recommended. Revascularization before noncardiac surgery is recommended in circumstances in which revascularization is indicated according to existing clinical practice guidelines.
5. Cardiac consultation may be helpful in determining which tests will be useful and in interpreting the results. The consultant can help optimize
the patient’s preoperative medical therapy and provide follow-up in the postoperative period. Such follow-up is crucial with the initiation of new drug therapies and often for patients with pacemakers and implantable cardioverter-defibrillator (ICD) devices (see section V-VI).
the patient’s preoperative medical therapy and provide follow-up in the postoperative period. Such follow-up is crucial with the initiation of new drug therapies and often for patients with pacemakers and implantable cardioverter-defibrillator (ICD) devices (see section V-VI).
D. Indications for preoperative coronary revascularization with either coronary artery bypass grafting or percutaneous coronary intervention (PCI) are in general the same as in the nonoperative setting. Surgery, in and of itself, is not an indication for coronary revascularization, regardless of extent of vessel disease or left ventricular dysfunction.
IV. PREANESTHETIC CONSIDERATIONS
A. Patients are likely to be anxious. Reassurance during the preoperative visit has been shown to be useful in decreasing anxiety. Anxiolytics may blunt rises in sympathetic tone and may be invaluable.
B. Cardiac medications are usually continued perioperatively. Possible exceptions include angiotensin-converting enzyme inhibitors (due to prolonged vasodilation), sustained-release or long-acting medications, and diuretics.
1. β-Blockers. The preponderance of evidence suggests that the perioperative administration of β-blockers is associated with a reduction in perioperative cardiac events but not surgical mortality. Preoperative β-blocker administration has been associated with an increased incidence of adverse effects such as bradycardia and stroke, especially when larger doses have been given. Patients who are on chronic β-blocker therapy preoperatively should continue to receive them in the perioperative period. In patients with moderate to high risk of perioperative myocardial ischemia or with three or more RCRI risk factors (e.g., diabetes mellitus, HF, coronary artery disease, renal insufficiency, cerebrovascular accident), it may be reasonable to start β-blockers before surgery. When possible, β-blockers should be started days to weeks before elective surgery and titrated cautiously. They should not be started on the day of surgery.
2. Statins. Patients taking statins should continue to receive statins perioperatively. Preoperative initiation of statin therapy is reasonable in patients undergoing vascular surgery as well as in patients with standard clinical indications for statin therapy who are undergoing elevated-risk procedures.
3. Aspirin. The efficacy of aspirin for the secondary prevention of MI in patients with ischemic heart disease has been well documented. Data on the risk of discontinuing antiplatelet therapy in patients with coronary stents have strongly suggested continuing aspirin in the perioperative period. See Chapter 1. The data on continuing aspirin in patients undergoing elective noncardiac, noncarotid surgery who have not had previous coronary stenting, however, are controversial. Some publications recommend that aspirin should not be stopped routinely in the perioperative period at all, while a recent randomized controlled trial concludes that aspirin has no significant effect on the rate of death or nonfatal MI but increases the risk of bleeding.
C. Timing of elective surgery in the setting of previous PCI presents a special challenge. Management decisions should be made in consultation with the patient’s cardiologist and surgeon.
1. Balloon angioplasty without stent placement. The ACC/AHA recommend that elective noncardiac surgery should be delayed 14 days after balloon angioplasty. Aspirin therapy should be continued in the perioperative period.
2. Bare metal coronary stents (BMS). Current recommendations are to delay elective noncardiac surgery for 30 days following PCI with BMS. This time period allows for the completion of thienopyridine therapy and the endothelialization of the stent. The risk of ischemic events is greatest within 30 days of PCI, significantly lower at 30 to 90 days, and lowest after 90 days. Aspirin therapy should be continued perioperatively.
3. Drug-eluting stents (DES). Thrombosis of DES can occur months after placement and is often related to the omission of thienopyridine therapy perioperatively. The current consensus recommendation is to defer elective surgery for 365 days following placement. Aspirin therapy should be continued perioperatively. Elective noncardiac surgery after DES implantation may be considered after 180 days if the risk of further delay is greater than the expected risks of ischemia and stent thrombosis.
4. Should a noncardiac surgical procedure be required within the time frame recommended for dual-antiplatelet therapy following PCI, consider continuing the therapy throughout the perioperative period. If bleeding risk necessitates the discontinuation of thienopyridine therapy, continue aspirin therapy and restart thienopyridines as soon as possible.
D. Supplemental oxygen should be provided to all patients who have a significant risk of ischemia.
E. Monitoring is discussed in Chapter 10.
F. Anesthetic Technique. No convincing outcome data support the superiority of one particular anesthetic technique over another in the management of patients at risk for perioperative cardiac events.
V. ISCHEMIC HEART DISEASE
Coronary artery disease afflicts an estimated 30% of patients undergoing surgery in the United States. CAD increases in prevalence with age. Other risk factors include hypercholesterolemia, male gender, hypertension, cigarette smoking, diabetes mellitus, obesity, and family history of premature development of ischemic heart disease. CAD is a risk factor for perioperative cardiac complications, including MI, unstable angina, congestive heart failure (CHF), and serious dysrhythmias.
A. Pathophysiology. Myocardial ischemia occurs when oxygen demand exceeds oxygen delivery.
B. Supply. The myocardium is supplied via coronary arteries. Myocardial oxygen supply depends on coronary artery diameter, LV diastolic pressure, aortic diastolic pressure, and arterial oxygen content.
1. Coronary blood flow is dependent on the aortic root-to-downstream coronary pressure gradient. Most coronary blood flow occurs during
diastole. Coronary artery blood flow in normal individuals is controlled primarily through local mediators. The coronary arteries of patients with significant CAD may be maximally dilated at rest.
2. Heart rate is inversely proportional to the length of diastole. Faster heart rates decrease the duration of maximal coronary perfusion.
3. Blood oxygen content is determined by hemoglobin concentration, oxygen saturation, and dissolved oxygen content. Increasing inspired oxygen fraction and/or hemoglobin concentration increases blood oxygen content.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree