Innervation of the birth canal
| Psychological | Antenatal education Pleasant environment |
| Complementary | TENS Aromatherapy Reflexology Acupuncture |
| Systemic analgesia | Nitrous oxide Inhalational IM opioids (pethidine) PCA (remifentanil) |
| Epidural | Bolus (either clinician or patient) Infusion +/- bolus |
Psychological methods
There is no doubt that adequate psychological preparation for labour significantly reduces pain. Breathing and relaxation techniques are taught routinely in antenatal classes. Attention to providing a non-clinical and stress-reducing environment is a key concern in midwifery-led units. Self-hypnosis can also be successful in the suitably motivated patient. Although still relatively uncommon, many mothers choose a water birth to aid with the pain of labour.
Complementary methods
Transcutaneous electrical nerve stimulation (TENS) can work well if introduced early enough in labour. The technique depends on the gate theory of pain transmission (as described by Melzack & Wall 1965). Stimulation must be applied to the dorsal columns of the spinal cord in the mid-thoracic region, above the level of the affected nerve roots, i.e. T10. TENS is effective early in labour but begins to lose its effect after 5 cm cervical dilatation is reached. Acupuncture has been advocated for use in labour but it is not in common use in the UK.
Systemic analgesia
Systemic administration of analgesic drugs remains popular in labour analgesia. Administration is usually by inhalation of nitrous oxide, or alternatively by intravenous or intramuscular administration of opioids.
Nitrous oxide is usually administered as a 50% mixture with oxygen (Entonox). Effective use of nitrous oxide depends on beginning the inhalation before the painful part of the contraction so that an analgesic concentration is reached by the time a contraction becomes painful, which is difficult to achieve. Subanaesthetic doses of sevoflurane (0.8%) have been shown to provide superior analgesia and patient satisfaction to nitrous oxide in the first stage of labour despite greater sedation. However, the use of volatile anaesthesia in labour has mostly been restricted to small-scale clinical trials.
Opioid analgesia may be given by either the intramuscular or the intravenous route. Local rules and preferences dictate the choice of opioid. Midwives may administer intramuscular opioids on their own responsibility, subject to local protocols relating to which drug, number of doses and accountability. Agents in widespread use include the agonists pethidine and diamorphine, while some units favour partial agonists such as meptazinol. All opioids have a reputation for causing fetal respiratory depression.
With the availability of remifentanil the intravenous route via a patient-controlled analgesia (PCA) device has witnessed a resurgence in popularity, particularly where logistical problems exclude the provision of a continuous epidural service. However, this unlicensed use of remifentanil in labour remains controversial. Although remifentanil appears to be a superior opioid it does have problems of itching and sedation with maternal desaturation and even complete apnoea. PCA devices may be simple ‘elastic recoil’ systems with a reservoir refilled at a fixed rate or complex computer-controlled devices with adjustable background infusions and lockout times.
Epidural analgesia
Epidural analgesia is the most effective form of labour analgesia, and remains the gold standard. However it requires the availability of a trained anaesthetist and is accompanied by rare but potentially major complications. Epidural analgesia comes into its own in long or complicated labours, malpresentations (such as occipitoposterior position or breech) and pre-eclampsia, where control of the blood pressure and improvements in placental blood flow are vital to the wellbeing of both mother and fetus. Indications for epidural analgesia are numerous, and contraindications are listed in Figure 5.3.
| Maternal refusal |
| Bleeding diathesis |
| Anatomical abnormality, some previous spinal surgeries |
| Hypovolaemia |
| Cardiac disease |
| Neurological disease |
| Sepsis, including local infection at injection site |
Contraindications to epidural analgesia
1. Maternal refusal is the only absolute contraindication to the technique. Anaesthetists in the UK are both morally and legally obliged to obtain informed consent for regional anaesthesia in labour. To continue, if the patient refuses after explanation of the risks and benefits, constitutes assault. It is helpful if methods of labour analgesia have been discussed before the event, either as part of an antenatal class or during a pre-anaesthetic assessment. Written information improves recall of any treatment discussion, as do tools such as the Obstetric Anaesthetists Association (OAA) epidural card. Evidence suggests that the pain and distress of labour does not preclude the ability of the mother to understand, retain information and make an informed choice. Robust estimates for rarer risks were produced by the NAP3 study. The incidence of permanent harm from central nerve blockade (CNB) in obstetrics was between 1 in 80,000 and 1 in 320,000. Local estimates produced by audit may help define more common and less serious risks. Not disclosing serious risks would be unacceptable. This discussion should be documented. It is worth adding at this point that wrong-route drugs errors appear to be more common in obstetric practice, so the anaesthetist should be particularly vigilant.
2. Bleeding diathesis. There is controversy about the provision of epidural and spinal blockade in patients with a bleeding tendency, whether this is congenital, such as haemophilia, or acquired, such as warfarinisation. It is generally accepted that any patient whose clotting times are more than 50% extended should probably not have an epidural (in practice this means an INR > 1.5). The potential problem is the vulnerability of the epidural venous plexus and concern that if an epidural vein is punctured there will be an uncontrolled bleed from it, causing a haematoma, which could compress the spinal cord. While this sequence of events is certainly possible, it is also exceptionally rare, with an incidence between 1 in 88,000 and 1 in 140,000.
The suitability of epidural analgesia for patients taking low-dose heparin or aspirin is also controversial. Aspirin causes a permanent block of platelet thromboxane A2, which affects any platelets in the circulation at the time the aspirin is administered. This continues for the life of any individual platelet, roughly 10 days from new. While this may appear to be a contraindication to epidural puncture, with its attendant risk of haemorrhage, the effect is quite limited because new, unaffected platelets are continually being produced. The best indicator of platelet function is the bleeding time, which suffers from poor reproducibility and a wide normal range. AAGBI have published explicit guidelines for epidural use and anticoagulation in their guide ‘Regional anaesthesia and patients with abnormalities of coagulation’ (2013). The risk is affected by clinical and pharmacological factors. In summary, the following limits do not increase the risk of epidural bleeding:
12 hours after a prophylactic dose of low-molecular-weight heparin (LMWH)
24 hours after a therapeutic dose of LMWH
4 hours after heparin (unfractionated)
An INR < 1.5 when on warfarin
NSAIDs without heparin
Aspirin without heparin
Platelet count > 75 × 109 L–1
In obstetric anaesthesia there are a number of other causes of acquired clotting failure, which may contraindicate epidural puncture. Pre-eclampsia is associated with deranged clotting function, particularly if severe or late in pregnancy. The platelet count will give an indication of the severity of the problem before it becomes clinically important. The HELLP syndrome (Haemolytic anaemia, Elevated Liver enzymes and Low Platelets) is a variant of pre-eclampsia. This condition is highly dangerous and causes extreme physiological upsets which can only be restored using the full facilities of an intensive care unit and haematological support. The pronounced coagulopathy that occurs in HELLP syndrome is a contraindication to epidural analgesia.
Intrauterine fetal death causes a failure of clotting as the fetus begins to macerate and breakdown products enter the maternal circulation. The process does not become significant until the fetus has been dead for more than about 5 days, but it is usual to measure clotting in this situation before an epidural is sited.
Placental abruption and other significant intrauterine bleeds may cause clotting failure secondary to the consumption of clotting factors in a vain attempt to stop bleeding from the spiral arteries. The spiral arteries pass through the myometrium to the placental bed and are usually closed off by uterine contraction after delivery. If the uterus is kept open by the presence of a fetus, blood clots or placental remnants the bleeding will continue until the uterus is emptied or the patient exsanguinates. The blood loss may not be seen as vaginal bleeding because it can remain concealed entirely within the uterus.
Amniotic fluid embolus causes a syndrome similar to major fat embolism, with clouding of consciousness, petechial haemorrhages, respiratory distress, hypotension and severe disseminated intravascular coagulation (DIC). This condition is rare but often fatal. Management consists of general supportive therapy and replacement of clotting factors and blood.
3. Anatomical abnormalities. Previous back surgery or spina bifida are relative contraindications to epidural analgesia because of difficulty in identifying bony landmarks or disruption of the normal anatomy of the epidural space. If the patient has had a lumbar laminectomy or spinal fusion then it is wise to check the level of surgery from previous notes or CT scans so that a level can be chosen for the puncture which has bony landmarks and avoids scar tissue or implanted metal. In spina bifida occulta, the landmarks are missing congenitally but the epidural space is relatively normal. The ligamentum flavum may, however, be absent. In the more severe forms of neural tube defect the epidural space and dural tube may be abnormal and so the situation is more complicated. None of the above situations is an absolute contraindication to epidural analgesia, but the technique may be difficult or impossible, the spread of local anaesthetic in the epidural space may be unpredictable, and the resulting pattern of block may be patchy or deficient.
4. Hypovolaemia must be corrected before any form of anaesthesia or analgesia, unless the situation is so dire that immediate surgery is the only way to reduce massive blood loss, in which case general anaesthesia will be indicated. In this situation, as with any hypovolaemic patient, induction must be carried out with extreme care. Sympathetic blockade caused by epidural or spinal anaesthesia in a hypovolaemic patient can have catastrophic consequences.
5. Cardiac disease is often taken as a contraindication to central neural blockade. In general, the effects of epidural anaesthesia are falls in both cardiac preload and afterload, which can be controlled by the cautious administration of local anaesthetic agents and by judicious use of fluids and vasoconstrictors. Pregnancy itself carries such major vascular and cardiac changes that it is difficult to reach term unless there are reasonable cardiac reserves. A growing number of patients with congenital cardiac disease, whether corrected or not, are being brought to term. In addition, the age of onset of major coronary artery disease is now falling within the childbearing years. These patients may be on the verge of major cardiac decompensation by the time they reach term. Each woman must be considered on her merits and ideally in consultation with a cardiologist. The backup of a cardiac surgical centre will be required very occasionally.
6. Neurological disease. Chronic neurological disease is often quoted as a contraindication to local or regional anaesthesia, without reliable evidence to confirm this view. Epidural/spinal anaesthesia is potentially dangerous in cases of raised intracranial pressure and should be discussed with a neurologist first. Spinal anaesthesia is contraindicated where there is suspicion of a tethered cord until suitable imaging is carried out. The height of the block and inclusion of opioids should be restricted if there is significant respiratory restriction due to underlying disease. Despite these caveats, most chronic neurological disorders, such as multiple sclerosis, follow a relapsing and remitting course, usually deteriorating at times of physical or mental stress and following a slow progression downward. Quality analgesia is even more important in this patient group. Childbirth is one of the stressful times likely to cause relapse, and if central nerve block is provided for analgesia or anaesthesia at this time then it is possible that any relapse will be linked to the technique, regardless of whether it is in fact to blame. Provided that this is explained and accepted by the patient, there is no reason why epidural analgesia should be withheld.
7. Sepsis. Systemic sepsis is a contraindication to epidural analgesia because of the cumulative detrimental affect on haemodynamic stability. Local infection at the proposed site of puncture is a relative contraindication, but there is usually a non-infected space available close by which can be used. Maternal pyrexia, however, should be taken as a contraindication because of the risk of blood-borne infection with a foreign body, the epidural catheter, in situ or a potential haematoma from epidural vein puncture providing an infective focus. Individual centres and clinicians may have different maximum maternal temperatures, above which they will not recommend epidural analgesia. Epidural analgesia limits maternal thermoregulation, and fetal death rate increases dramatically in maternal pyrexia, so it may be preferable to avoid the use of epidural anaesthesia in this situation.
Epidural technique
Detailed knowledge of the relevant anatomy is essential (Figure 5.4). For further details of epidural technique, see Chapter 7.
| Boundaries | Superior | Closed at foramen magnum |
| Inferior | Closed at sacrococcygeal membrane | |
| Anterior | Posterior longitudinal ligaments, vertebral bodies | |
| Posterior | Vertebral laminae, ligamenta flava | |
| Lateral | Open, pedicles and intervertebral foramina | |
| Shape | Broadly triangular, apex posteriorly | |
| Contents | Veins, arteries, fat, lymphatics, nerve roots and dural cuffs | |
The epidural space is a potential space within the spinal canal. It is broadly triangular in shape with the apex posteriorly. The shape varies considerably from level to level, being more oval in the neck. Superiorly it is closed at the foramen magnum, where the spinal dura mater and the periosteum of the spinal canal fuse to form the intracranial dura mater. It is, therefore, impossible for a true epidural injection to extend intracranially. Inferiorly, the epidural space is closed at the sacrococcygeal ligament. The anterior boundary lies within the spinal canal, being formed by the posterior longitudinal ligaments of the spinal column, the vertebral bodies and the intervertebral discs. Posteriorly the space is bounded by the ligamenta flava (these may be paired at each level or one pair of continuous ligaments – opinion varies) and the vertebral laminae. There may be a plane of cleavage between the ligamenta flava that can give an impression that there is no ligament when a needle passes through it. Laterally the epidural space is bounded by the pedicles and laminae of the vertebrae and by the intervertebral foramina, and thus it is not a closed space laterally. The space contains the spinal dural sac and its contents: the spinal cord and nerve roots. The epidural space itself contains fat, arterioles, a complex of thin-walled valveless veins which drain into the azygos system, lymphatics and the spinal nerve roots after they cross the dura and before they exit through the intervertebral foramina. The nerve roots carry with them a cuff of dura that may extend out into the paravertebral space.
To reach the epidural space from the skin of the back, the tip of the needle must pass through successive layers of tissue. The bones over the lumbar area are palpated and the spaces between spinous processes identified. Once a suitable space has been identified (L2/3 or L3/4 are usually the easiest and most consistent to use) the needle is inserted through the skin, staying strictly on the midline, although a deliberate paramedian approach is acceptable. The first ligament encountered is the supraspinous ligament. This has a ‘crunchy’ consistency and is up to 1 cm thick. The interspinous ligament feels ‘spongy’ and can be up to 6 cm thick. The ligamentum flavum is very variable in thickness, up to 2 cm, but is usually tough and difficult to penetrate. The essence of the technique is that with the needle tip in the ligamentum flavum, nothing can be injected through the needle whereas after careful advancement the tip will emerge into the epidural space and there will be a sudden total loss of resistance to injection.
Ultrasound-guided epidural needle placement
Ultrasound guidance is increasingly being used, both to perform epidural insertion and in the assessment of the difficult spine. It can provide information regarding position of midline structures, depth of epidural space and angle of needle entry. This may be particularly useful when planning epidural placement in the obese patient. Some evidence suggests that it may enhance learning of epidural placement, reduce the number of failed attempts and help with the management of failed catheter placement.
Combined spinal–epidural in labour
Combined spinal–epidural (CSE) techniques may be used for analgesia in labour. An epidural needle is sited in the epidural space and a longer small-gauge spinal needle is typically passed through it. A small dose of a mixture of bupivacaine and fentanyl is injected into the subarachnoid space and the spinal needle is then withdrawn and an epidural catheter passed through the epidural needle. The spinal solution establishes rapid analgesia, providing good blockade of the sacral nerve roots while lessening maternal and umbilical cord concentrations of local anaesthetic. Spinal doses of 2.5 mg of local anaesthetic appear to provide satisfactory analgesia. The epidural catheter is used for further doses during the second stage of labour. The patient is allowed some mobility while the spinal solution is effective but this may be limited by the need for continuous monitoring of the fetus. Each unit must have written policies to establish the limits to mobility in labour.
Epidural test doses
It is impossible to be absolutely sure of the correct placement of an epidural catheter until a dose of local anaesthetic agent has been injected. A test dose serves two purposes, first to identify vascular placement and second to identify intrathecal placement. To achieve this, a test dose must be small enough to do no harm if in the wrong place but large enough to show an effect. Departments should have their own policies for ‘testing’ the epidural, and it is helpful if all practitioners follow the policy to provide consistency on what constitutes a ‘failed’ block. Many practitioners use a 3 mL dose of either 0.5% or 0.25% plain bupivacaine. There are advocates for both adrenaline-containing and dextrose-containing test doses but neither is in popular use.
In practice, 3 mL plain isobaric bupivacaine, placed directly into the CSF, will produce total spinal anaesthesia within 5 minutes. Hyperbaric bupivacaine (heavy Marcaine) will have a less extensive result, and if it is placed intravenously there will be no noticeable effect. Larger volumes of local anaesthetic agent injected into the lumbar epidural venous plexus tend to pass backwards up the basilar vessels and cause a short-lived loss of consciousness or at least a period of light-headedness with lingual and circumoral paraesthesia. The rationale behind using an adrenaline-containing solution is that, on intravenous injection, there will be a measurable increase in heart rate. While this may be so, the increase so caused will be within the pulse-rate variation of any woman in labour and so may not be distinguished from normal.
Having given the test dose and waited an appropriate time for an effect to appear, usually 5 minutes, the main dose may be given. Traditionally the choice was 0.25% or 0.5% plain bupivacaine by bolus top-ups of between 6 and 10 mL as necessary to relieve the pain every 30 minutes. The top-ups may be given all in one position, usually semi-reclining, or half the dose may be given in each lateral position with 5 minutes between. This method has largely been superseded by a variety of low-dose infusion and patient-controlled epidural analgesia (PCEA) techniques that have the advantage of reducing both the drug load and the unwanted effects of the traditional epidural, such as high-density motor block and an increased incidence of instrumental delivery.
The majority of infusion/PCEA epidurals begin with a single top-up to rapidly establish the analgesia before commencing the infusion. The bupivacaine/fentanyl combination appears to increase both the analgesia and the penetration of the block without any obvious drawbacks. Recent evidence suggests that there are no measurable fetal effects of the opioid at doses in current use, although addition of fentanyl clearly increases symptomatic itching in the mother. No single regime for epidural injection has gained wide acceptance. Infusion regimes vary considerably, but most are based on either 0.1–0.15% bupivacaine or 0.2% ropivacaine, each with 1–5 μg mL–1 of fentanyl. Bolus doses in a PCEA may be 5 mL of 0.1–0.15% bupivacaine with or without 2 μg mL–1 fentanyl followed by a lockout of 10 minutes. PCEA also allows the patient control over her analgesia, reducing breakthrough pain while waiting for a midwife/clinician-delivered bolus. Each unit should have a single standard mixture and infusion/bolus protocol with which all anaesthetists and midwives should be familiar.
Complications
Epidural anaesthesia carries a risk of complications, the more major of which are listed in Figure 5.5.
1. Dural tap and post-dural puncture headache (PDPH) occurs in about 0.5–1.5% of obstetric epidurals. The majority of these will become symptomatic. Immediate treatment involves establishing a working epidural at another lumbar interspace. The patient, midwifery and obstetric team should be notified so that a prolonged second stage can be avoided. An elective forceps or instrumental delivery may be required. The differential diagnoses for postpartum headache are shown in Figure 5.6.
PDPH is typically postural and improves on lying down. It usually presents within 72 hours and is associated with pain in the frontal-occipital region, neck stiffness with visual and auditory symptoms. The patient should be reviewed daily by a senior member of the anaesthetic staff. The pain should initially be treated by encouraging oral fluids and by simple analgesia. If the headache becomes incapacitating, an epidural blood patch (EBP) remains the gold-standard therapy. After obtaining consent this would typically be carried out between 24 and 48 hours after the original dural puncture. Infection, pyrexia, refusal and coagulopathy will contraindicate the technique.
Under aseptic conditions a new epidural puncture is carried out at or below the site of the original puncture and up to 20 mL of the patient’s blood is injected into the epidural space. This technique should be performed with two scrubbed anaesthetists. It has been routine to send further blood for culture, although the results are usually negative. The patient is usually advised to lie still for 1–2 hours afterwards before gently mobilising. Initial success rate for EBP is high, but many women (possibly up to 40%) may have a recurrence of headache requiring further treatment. Patients should be informed to immediately report pyrexia, back pain or radicular pain if it occurs after EBP. It is always worth reassessing the patient for an alternative diagnosis if she does not respond as expected to treatment.
2. Intravascular injection. While proper use of test doses should identify intrathecal and intravascular injections at that stage, epidural catheters can migrate into vessels or across the dura at any stage during the conduct of the technique, and the full dose of local anaesthetic may be inadvertently injected into the circulation. Clinical features and management are covered under Local anaesthetic toxicity in Chapter 7.
Injection of local anaesthetic into the epidural veins may only cause paraesthesia of the tongue and lips but can also cause agitation, sudden loss of consciousness and seizures as the local anaesthetic agent affects the brain. Cardiovascular collapse may quickly follow. Advanced life support (ALS) should be delivered and an infusion of Intralipid commenced. The airway and respiration must be adequately maintained, and tracheal intubation and controlled ventilation may be necessary. Once this has been achieved the circulation must be supported by fluids, vasopressors and cardiac massage if appropriate according to standard ALS protocols.
3. Intrathecal injection. The effects of intrathecal injection may be slower in onset but no less of a problem than intravascular injection. Progressive rising paralysis of the whole body, including the muscles of respiration, occurs, accompanied by a significant fall in blood pressure. The feature which distinguishes intrathecal injection from massive epidural is the onset of cranial nerve effects, particularly facial paralysis, trigeminal anaesthesia and rapid loss of consciousness. As with intravenous injection, the airway and respiration must be addressed first, followed by the circulation as dictated by ALS techniques.
These rare but major problems are the reason for direct observation of the patient for 20 minutes after an epidural injection or top-up.
4. Neurological complications of epidural analgesia are extremely rare and usually relate to cauda equina syndrome if there has been local neural toxicity by either too high a concentration of adrenaline in the injected drug or, more likely, the wrong drug administered. The majority of neurological complications following childbirth are related not to epidurals but to the management of labour, particularly where a large fetus has become obstructed in the second stage of labour for a prolonged time. This scenario results in compression of the roots and trunks of the lumbosacral plexus within the pelvis, especially L1 as it passes over the brim of the true pelvis. The most common defects subsequently are foot drop (lateral peroneal nerve), sciatic palsies or femoral nerve palsies. Neurological symptoms should be thoroughly documented at time of presentation and a referral made to a suitable clinician. Many symptoms will resolve slowly over time but represent a significant concern for patients. Each case should be managed with due care and appropriate follow-up.
5. Backache. Epidural analgesia in labour has developed a reputation for causing low-grade but persistent backache after delivery, which is probably related not to epidural analgesia itself but to the management of the back in labour. In the absence of pain sensation, proprioception and muscle tone to protect the joints and ligaments of the back, there is a possibility of musculoskeletal strain. Suitable imaging and specialist neurological input will exclude significant pathology.
| Dural tap |
| Intravascular injection |
| Intrathecal injection |
| Neurological |
| Backache |
| Pressure areas |
| Migraine |
| Meningitis |
| Post-dural puncture headache (PDPH) |
| Cortical vein thrombosis |
| Simple/tension headache |
| Subarachnoid haemorrhage |
| Pre-eclampsia/eclampsia |
| Space-occupying lesion (tumour, haemorrhage) |
| Posterior reversible leucoencephalopathy syndrome |
| Cerebral infarction/ischaemia |
| Sinusitis |
Operative anaesthesia
Anaesthesia for operative surgery in obstetrics falls into two main areas. First, operative delivery, for example Caesarean section and forceps procedures; second, post-delivery procedures such as retained placenta. Both are amenable to being carried out under regional or general anaesthesia. In the case of retained placenta, before considering a regional technique (such as spinal anaesthesia) which removes sympathetic tone and causes profound vasodilatation, it is essential to first accurately assess blood loss and restore circulating volume.
Regional anaesthesia
Central neural blockade for operative obstetric anaesthesia requires a different approach to the provision of analgesia in labour. In labour the only essential feature is analgesia – motor block is a distinct disadvantage. For obstetric surgery, including forceps delivery, removal of retained placenta and Caesarean section, complete anaesthesia of the relevant area is necessary. In labour the highest dermatome required is T10, whereas for Caesarean section the upper limit needs to be a minimum of T6, though there is still some debate about whether it should be even higher than this (T4) to adequately cover the variable innervation of the peritoneum. The sacral nerve roots also need to be blocked. Light touch is the modality that shows the least variability when assessing level of block. The dose of local anaesthetic agent necessary to achieve this at term is only about two-thirds of that required in the non-pregnant patient for a comparable result.
Spinal anaesthesia for obstetrics offers advantages over epidural anaesthesia because of speed of onset and intensity of block, but disadvantages because of severity and speed of onset of hypotension and the less adjustable nature of the technique, unless a combined technique is used. The major disadvantage of spinal anaesthesia in obstetric anaesthesia has always been the incidence of post-dural puncture headache (PDPH). This has been significantly reduced by the use of solid-tipped (non-cutting) needles with side holes such as the Sprotte and Whitacre point needles. The only spinal solutions with current product licences are 0.5% hyperbaric bupivacaine and 0.5% plain levobupivacaine. This latter solution is slightly hypobaric. The optimum dose for establishing an effective block (i.e. no intraoperative pain for 90–99% of parturients) remains the topic of much research.
There has been much interest in recent years in ‘low-dose’ spinal techniques for Caesarean section, and they have indeed become successful standard practice in some units. The definition of what constitutes a low dose varies, but the ED95 for 0.5% heavy Marcaine is 2.2 mL or 11 mg. The addition of an opioid (fentanyl/diamorphine) has definite advantages in extending both the duration and quality of block. Low-dose regimes have the advantage of less maternal hypotension and reduced motor blockade. However, they carry with them a slower onset and shorter duration of blockade and increased frequency of intraoperative pain and potential increased risk of conversion to general anaesthesia. They should only be used in experienced hands in obstetric units with appropriate operating conditions and the ability to supplement analgesia through an epidural catheter as part of a combined spinal–epidural anaesthetic. This combines the speed of onset and intensity of spinal anaesthesia with the adjustability and duration of epidural anaesthesia. Spinal anaesthesia can be extended by injection of saline into the epidural catheter shortly after the spinal dose, as this increases pressure in the epidural space.
For obstetric surgery under epidural anaesthesia alone, a catheter is inserted in the usual way and, after a test dose, a main dose of local anaesthetic agent is given. This may be given as one dose or in divided doses. The local anaesthetic agent of choice should ensure rapid onset of an intense block with a duration of action in excess of 1 hour. Up to 30 mL of bupivacaine 0.5% or 20 mL of 2% lidocaine, both with 100 μg fentanyl, usually produces satisfactory blockade. The fetus should be monitored during epidural top-up and the anaesthetist should remain with the mother. If a top-up is required quickly, 20 mL lidocaine 2% with 0.1 mL of 1:000 adrenaline, 100 μg fentanyl (and 1 mL 8.4% bicarbonate) may be used.
Every patient should be assessed for the risk of venous thromboembolism, and if at risk a dose of low-molecular-weight heparin should be given 4 hours after the regional block is established or the epidural catheter removed. This should not be delayed unless there is a good reason, such as haemorrhage, coagulopathy or traumatic bloody tap. Placement or removal of an epidural catheter should occur at least 12 hours after a prophylactic dose of LMWH.
The prevention and management of hypotension as a result of central neural blockade falls into two areas: volume loading and vasopressors. Volume loading before the administration of the block requires administration of 500–1,000 mL of crystalloid solution or 500 mL of colloid solution. Traditionally ephedrine was used in small intravenous doses (3–6 mg) to correct hypotension. Ephedrine crosses the placental barrier and may thus cause a fetal tachycardia and acidosis. Prophylactic phenylephrine infusions and fluid co-hydration have made maternal hypotension much less frequent. Phenylephrine does not cross the placenta.
In the preoperative period the patient should be warned that regional anaesthesia may not give total loss of sensation. Pain during Caesarean section is a common reason for complaint and litigation. Clear honest documentation pertaining to the consent, the assessment of the block (two modalities, cold and light touch) and the management of intraoperative pain are clinical and medico-legal standards.
General anaesthesia
Obstetric general anaesthesia is usually considered to be one of the higher-risk subspecialties of anaesthesia because of the potential for urgency, uncontrolled bleeding and aspiration of gastric contents. In fact, as a cause of maternal mortality, anaesthesia ranks very low (0.31 deaths per 100,000 maternities), and deaths due to amniotic fluid embolism and obstetric haemorrhage are twice as common. This low mortality is not a reason for complacency, but a result of sustained work in eliminating the main causes of anaesthetic-related problems by intensive training in managing difficult and failed intubation, and universal antacid prophylaxis. Sadly, deaths due to failure to ventilate the lungs and postoperative aspiration still occurred in the most recent Confidential Enquiry into maternal and child health.
As a rule of thumb, there is no such thing as a pregnant woman with an empty stomach. The gastric contents also tend to be more acid than in the non-pregnant woman. Progesterone-induced relaxation of the lower oesophageal sphincter, along with the higher intra-abdominal pressure in late pregnancy, tends to encourage the regurgitation and aspiration of gastric contents into the trachea. While Mendelson actually described obstruction to respiration by solid matter, the aspiration of liquid and the resulting chemical pneumonitis are usually called Mendelson’s syndrome. Acid aspiration in pregnancy causes a gross chemical pneumonitis, which distinguishes it from the aspiration pneumonia of the non-pregnant. Routine antacid prophylaxis in the delivery suite reduces both the volume and the acidity of gastric contents. Common regimes involve administration of regular oral H2 receptor antagonists to all admissions to the delivery suite and 0.3 molar sodium citrate solution when a decision is made to proceed to surgery. Variations on this theme include the administration of intravenous ranitidine and metoclopramide, the latter to encourage gastric emptying, although this effect is difficult to show and variable. Magnesium trisilicate mixture is little used now because it is particulate and does not mix well with gastric contents.
In the second and third trimesters of pregnancy tracheal intubation is considered mandatory because of the potential for acid aspiration. For the same reasons rapid sequence induction (RSI) with cricoid pressure is also necessary. The hormonal changes of pregnancy, as pertaining to intestinal function, remain for some 48 hours post partum, and so it is wise to apply RSI for up to a week after delivery. The standard general anaesthetic technique involves a wedge under the right buttock to displace the uterus from the inferior vena cava, RSI with thiopental and suxamethonium (propofol has no licence for use in late pregnancy, although it is increasingly used). Tracheal intubation should be followed by controlled ventilation of the lungs with 50–70% nitrous oxide and a volatile agent of choice. Suitable muscle relaxants include atracurium and vecuronium. Mivacurium should be used with care because of the reduced activity of plasma cholinesterase in late pregnancy, which may delay its offset. At the end of the procedure the tracheal tube should be removed with the patient in the lateral position, head down. The recovery period can be particularly dangerous in the postpartum patient and extubation should be considered only after the return of protective reflexes.
Tracheal intubation in the pregnant woman can be notoriously difficult. The anatomy of the chest changes in pregnancy, with an increase in functional residual capacity (FRC) giving an impression of hyperinflation. This is combined with an increase in the size of the breasts and an apparent shortening of the neck (because of the increase in FRC). Note that the majority of pregnant women have a full set of natural teeth.
Pre-eclampsia may cause laryngeal oedema, and it is now recognised that the Mallampati score may change as labour progresses, causing further difficulties. Endotracheal tube size should be reduced in expectation of difficulty. While the laryngeal mask does not provide sufficient barrier to gastric contents for routine use, in a case of failed tracheal intubation it may have a role (see Chapter 2).
Equipment for the management of difficult intubation must be available at all times and within arm’s reach whenever obstetric anaesthesia is practised. Desirable equipment includes a range of laryngoscope blades and handles, including a polio blade, a variety of tube sizes down to 6.0 mm, and stylets and bougies to aid intubation. A method of transtracheal ventilation should also be readily available and staff trained in its use.
Accidental awareness is more commonly encountered in obstetric general anaesthesia than in any other specialty. The cause is usually either failure to introduce sufficiently high concentrations of vapour early enough, before the brain concentration of the induction agent begins to fall, or failure to maintain sufficiently high concentrations of vapour and nitrous oxide throughout the procedure.
Where death has occurred after difficult or failed intubation, the cause has not been the failure to intubate but the failure to oxygenate the patient between attempts. This point cannot be emphasised enough. Regular emergency drills (possibly involving simulation) and familiarity with equipment in managing the difficult and failed airway will help to eliminate the problems of human behaviour involving fixation and denial when faced with an emergency scenario.
Non-obstetric surgery in the pregnant patient
When to consider a patient pregnant
As a general rule, the risks of acid aspiration begin to outweigh the risks of tracheal intubation at about 14–16 weeks gestation, and from this time onwards the patient should be considered as an obstetric problem. At this stage anti-acid prophylaxis is sensible. The hormonal changes of pregnancy fade rapidly after delivery, along with the effects on gastric function, and so it is probably safe to revert to non-pregnancy anaesthetic techniques at about 1 week post partum, although purely elective surgery may be delayed longer.
Non-obstetric surgery
It must be remembered that the pregnant patient may present with a variety of other surgical and medical pathologies. Common presentations include appendicitis, trauma and malignancy. Pathology or intra-abdominal surgery increases the risk of miscarriage, preterm labour and fetal demise. Timing of non-emergency surgery is important and will affect both the miscarriage rate and the chances of abnormal fetal development. The miscarriage rate after surgery in the first trimester may be as high as 10%.
The anaesthetist must take into account the health of both the mother and the developing fetus, although in an emergency the mother’s life takes priority. The trimester will dictate which physiological changes are relevant to the anaesthetist. But a fundamental respect for the increased cardiac output, oxygen demand, aortocaval compression, aspiration risk and airway difficulties should always be held. Rapid sequence induction is recommended in the second trimester. MAC values are slightly reduced in pregnancy, but all volatile anaesthetics cause uterine vasodilatation and relaxation, increasing the risk of uterine bleeding.
Risks to the fetus can be minimised by early recognition of disease and consideration of placental transfer of drugs, teratogenicity and maintenance of uteroplacental perfusion, both in terms of oxygenation and flow (Figure 5.7). Maternal PaCO2 should be kept normal to avoid fetal acidosis. Nitrous oxide should be avoided in the first trimester because of teratogenicity. Extubation should occur in the full lateral position with the patient fully awake.
It is worth remembering that no particular anaesthetic technique has been shown to be better than another in reducing the risk of surgery. The risk of pregnancy loss and other problems should be discussed during the consent process. Thromboprophylaxis should be considered.
The patient, an obstetrician and a midwife should be involved in discussions regards clinical management, and where possible the fetal heart should be monitored before and after the case.
Cell salvage in obstetric care
Obstetric haemorrhage is still one of the leading causes of maternal mortality in the UK. Cell salvage has the potential benefit of reducing transfusion infection risk (although salvaged blood may still be infected). It also avoids anaemia (which may occur in normovolaemic dilutional and preoperative autologous donation methods) and can be used in an emergency. It may be acceptable to those who have religious objections to receiving transfused blood products – although individual consent is still vital. It is recommended that the cell salvage machines be used whenever there is a significant risk of maternal haemorrhage (multiple previous sections, multiple pregnancies, LSCS at full dilatation, abnormal placentation etc.) or if allogenic blood is unavailable or unsuitable. Trained staff should be involved in order to reduce operator error.
Obstetric haemorrhage is unpredictable. The cell salvage machine can be set up in standby mode, consisting of simply the suction and reservoir apparatus. It can then be converted to filter blood if the need arises. The main concern in obstetric care has been the risk of alloimmunisation of RhD blood and the potential for reinfusion of amniotic fluid causing an amniotic fluid embolism (AFE). Anti-D can be used to treat reinfused rhesus-mismatched fetal erythrocytes. AFE is now considered an anaphylactoid type reaction. The incidence of AFE is reduced by having two suction systems, processing only whole bowls and using a leucodepletion filter (Figure 5.8). The second reservoir is only filled once the fetus has been delivered and the amniotic fluid removed into the waste. It has been reported that leucodepletion filters may produce hypotension due to a biochemical reaction as well as physically slowing down the rate of reinfusion. Despite the risks (Figure 5.9), no single complication that led to an adverse outcome has been exclusively attributed to cell salvage, in over 800 published cases. It is of course possible that the risk is lower than this.
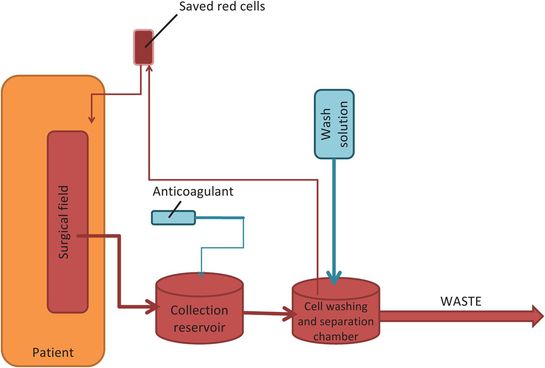
Cell saver system
| Infection (bacterial, viral) |
| Amniotic fluid embolism (very rare) |
| Hypotension |
| Disseminated intravascular coagulation |
| Transfer of fetal RBCs and maternal alloimmunisation |
| Heparin toxicity |
| Red blood cell lysis |
| Potential sickling of transfused blood in sickle cell patients |
The paediatric patient
This section is predominantly concerned with children of 20 kg or more in weight. In practice, this will usually equate to an age of about 5 years. A convenient formula for estimating the weight of a child is:
Assessment
Preoperative assessment in children should be as rigorous as in adults, and questions should be addressed to the child even though the parents may answer for them. Most children are healthy, but chronic conditions such as asthma, multiple allergies, congenital heart disease and systemic conditions (such as muscular dystrophy) may also be encountered. The presence of one congenital abnormality should stimulate the search for others. Chromosomal abnormalities may be linked, particularly with congenital heart disease. Except for true emergency surgery, children with colds or upper respiratory tract infections should have their surgery cancelled and rescheduled to a later date. The inflamed airway is exquisitely sensitive to any kind of manipulation, resulting in laryngeal spasm. Laryngeal spasm in children is particularly dangerous because of the rapid onset of severe desaturation, made more marked by their higher metabolic rate.
There is no universally good premedicant for children. Oral midazolam (0.5 mg kg–1, maximum 20 mg) usually works well. The IV dose can be given orally and its taste disguised in a small amount of clear juice. It usually takes 20–30 minutes to reach peak effect, and administration should be timed accordingly. The duration and effect of oral midazolam is sometimes unpredictable and it can cause paradoxical excitation. Day-case admission can result in insufficient time for anxiolytic premedication to take effect (and the use of sedatives in day surgery may be undesirable). All children should have topical local anaesthetic cream or gel applied to the proposed venepuncture site at least 1 hour before anaesthesia. Drug doses in children should always be calculated on a weight-related basis, a calculation which will give an approximation of the required dose. If dilution of a drug is proposed then each syringe should be labelled with the drug name and concentration. Ambiguity must be avoided at all costs.
Equipment
Anaesthetic equipment for patients under 20 kg body weight is quite specialised, and there is no gradation to adult equipment. For all paediatric patients the breathing system dead space and resistance should be kept to a minimum by avoiding catheter mounts, angle pieces and valves. Controlled ventilation may be preferable because of the inevitable increase in dead space after induction of anaesthesia and the increased work of breathing. The Mapleson E or F system is preferable for patients less than 20 kg, but it can also be used for heavier patients if the volume of the expiratory limb is more than the calculated tidal volume and the fresh gas flow for spontaneous ventilation is more than 2.5 times minute volume. Tidal volume approximates to 8 mL kg–1 in the child, and a respiratory rate of about 20 per minute is usual. If ventilation is to be controlled then a minute volume divider type of ventilator should only be used for tidal volume settings greater than 300 mL. The reason for this is that below this the ventilator becomes inaccurate in delivery because of the higher proportion of compressible volume related to total tidal volume. Many modern integrated ventilators will ventilate tidal volumes down to 50 mL, although with tidal volumes less than 300 mL either a T-piece occluder type system should be used, or a Mapleson D with a ventilator such as the Nuffield Anaesthesia Ventilator Series 200 (with Newton valve for neonates or premature babies).
If tracheal intubation is proposed then account should be taken of the increased resistance to breathing that this introduces. The resistance to flow in a tube is inversely related to the fourth power of the radius, and so halving the diameter will increase resistance by 16 times. Any tracheal tube will have a smaller internal diameter than the natural airway, particularly if the tube is cuffed. Unless there is a risk of tracheal soiling, uncuffed tubes are preferable because of the larger internal diameter that this allows. The anatomy of the child larynx is different from the adult (Figure 5.10). In consequence, the use of cuffed tubes in the under-10 age group renders the subglottic region vulnerable to oedema, particularly if an overly large tube is introduced with force. That said, there has been a recent shift to using cuffed tracheal tubes in younger children. Even small amounts of secretion in a tracheal tube will significantly increase the resistance to gas flow.
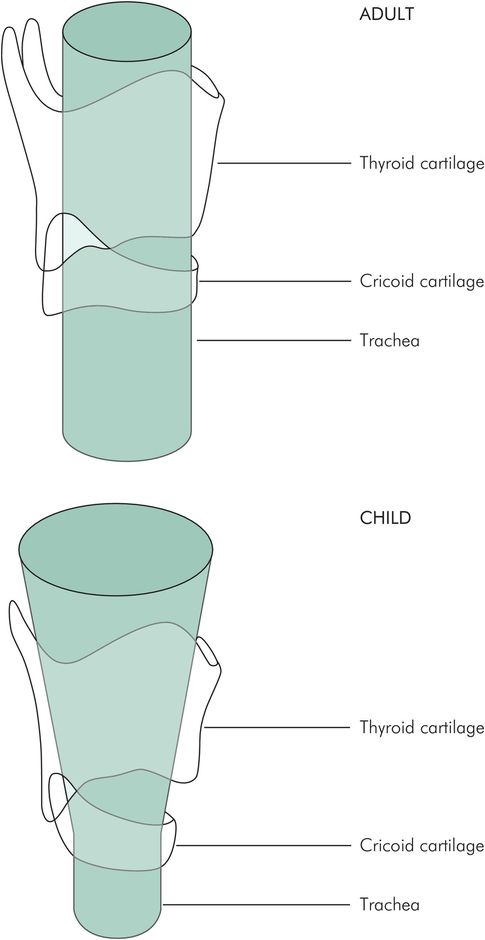
Anatomy of the infant larynx
Figure 5.11 offers guidance in determining the appropriate size of endotracheal tube for use in children.
| ETT internal diameter (mm) = age/4 + 4 = tube diameter mm (neonate size 3–3.5) |
| Oral ETT length (cm) = age/2 +12 |
| Nasal ETT length (cm) = age/2 +15 |
Fluid therapy
The fluid choice will depend upon the age of the child and the nature of fluid loss. Fluid requirements should take into account maintenance fluid, deficit and ongoing losses. Resuscitation in shocked states should usually be with isotonic normal saline or Ringer’s lactate (10 ml kg–1). Blood sugar should be checked and glucose and potassium given as appropriate. Hypotonic solutions in particular can cause significant problems with hyponatraemia in paediatric patients.
Intravenous fluids in children should be given via a burette giving set or a volume-controlled pump and should always be calculated on a weight-related basis (Figure 5.12). A minimum of 2 mL kg–1 per hour should be given to those not receiving oral fluids. Blood replacement can be very difficult to calculate, and is based on replacing loss for loss when 10% blood volume has been spilt. Losses are assessed by swab weighing and accurate suction measurement. Blood volume may be estimated as 80 mL kg–1 in small children, falling to 70 mL kg–1 in adults. Blood loss of 8 mL kg–1 will, therefore, need replacing during surgery.
| Weight | Rate (mL h–1) |
|---|---|
| 0–10 kg | 4 per kg of body weight |
| 11–20 kg | 40 + (2 per kg over 10 kg) |
| ≥ 21 kg | 60 + (1 per kg over 20 kg) |
| For example: Age 7 Weight = 7 + 4 × 2 = 22 kg Fluids = 40 + 20 + 2 = 62 mL h–1 | |
Analgesia
Much of the surgery carried out on children lends itself very well to regional anaesthesia, and this method provides very high-quality early postoperative analgesia. Caudal epidural analgesia is particularly well suited to lower abdominal and lower limb surgery and is easier to perform in children than in adults. However, it should be noted that meticulous care must be taken with indwelling epidural catheters postoperatively. Paediatric patients may not express concerns regards new symptoms, e.g. new motor block, as an adult would. Most children over 6 years of age can use a PCA machine, and a nurse-controlled version is also available (NCA). Other useful preparations involve opioid lollipops and oral preparations (such as Oramorph). Rectal administration of non-steroidal agents (such as diclofenac) and paracetamol remain popular. Figure 5.13 shows paediatric doses of analgesic drugs.
| Drug | Paediatric dose |
|---|---|
| Paracetamol | Max 90 mg kg–1 day–1 in 4 divided doses (max 2 g day–1 if under 50 kg) First dose 20 mg kg–1 Subsequent doses 10 mg kg–1 at 6-hourly intervals |
| Ibuprofen | 5 mg kg–1 every 8 hours (max 400 mg day–1) |
| Diclofenac | PR 1 mg kg–1 every 16 hours (max 150 mg day–1) |
| Codeine | 1 mg kg–1 every 4–6 hours |
| Morphine | PO 0.4 mg kg–1 every 2–4 hours IV 0.1 mg kg–1 every 2–4 hours PCA (1 mg kg–1 in 50 mL normal saline, bolus 1 mL, 5-minute lockout, max 1 mg mL–1) |
Specialist surgery
General and urological surgery
Elective general and urological surgery in childhood tends to be restricted to herniotomy, orchidopexy and circumcision, all of which can be carried out on a day-case basis with a combination of general and regional anaesthesia. Appendicectomy is another frequent operation in children and is always an inpatient (emergency or urgent) procedure. Groin incisions can be covered very well by inguinal field blockade, while penile block is particularly good for circumcision. In contrast, the more widespread and bilateral anaesthesia of a caudal block may restrict mobility and be complicated by urinary retention, particularly in larger children. Oral analgesia should be given before the effect of the local anaesthetic wears off. For appendicectomy, an RSI technique should be used. Wound infiltration with local anaesthetic provides some postoperative pain relief. While some of these children are relatively well, others are pyrexial and toxic, particularly if the diagnosis is made late. These latter need to be managed very carefully and their state of hydration properly assessed and corrected in the preoperative period.
Orthopaedic surgery
Much of the orthopaedic surgery in this age group is the result of trauma. A full skeletal survey should be carried out so that other injuries are not missed, particularly head injuries and cervical spine injuries. Blood loss and analgesia requirements should receive particular attention before anaesthesia. After trauma, patients are usually assumed to have a full stomach and should be managed as such, with preoxygenation and RSI techniques. Postoperative analgesia for fractures and soft tissue injuries is best provided by regional anaesthesia, although there remains a debate about the development of compartment syndrome being masked if there are forearm or lower leg fractures. Elective orthopaedic procedures in children aged 5 years and over differ little in their anaesthesia requirements from those in adults.
Dental and ENT surgery
The core of both dental and ENT anaesthesia is the problem of the shared airway. In both types of surgery the anaesthetist and the surgeon need good access to a clear airway kept free of debris and blood. This has resulted in the development of special equipment and techniques for this situation.
Dental surgery
In the main, dental surgery in children is restricted to simple dental extraction. In some areas of the UK dental caries is still a common indication. Elsewhere, with improvements in dental hygiene and fluoridation of water, the indication has changed but the procedure has not. As a result children more often present because of crowding within the mouth or congenital dental problems. The Poswillo report (Poswillo 1990) has resulted in dental extraction under general anaesthesia being carried out almost exclusively in hospitals. They must be licensed and carry the full range of monitoring and resuscitation equipment as well as trained anaesthetic staff
Surgery is typically carried out on a ‘walk in, walk out’ basis, and day-case rules should be applied rigidly. Preoperative assessment is carried out as usual, with particular reference to fasting and respiratory conditions (such as asthma). There is little opportunity for preoperative investigation or correction of abnormalities. During the preoperative visit a discussion with the child and guardian is vital. This gives the opportunity to explain the induction of anaesthesia and expectations for recovery. It is useful to add at this point that the child should not be restrained by theatre staff. A frank discussion should allay most fears, help to gain trust and reduce the number of children who simply run away!
Induction of anaesthesia may be intravenous or inhalational, but venous access should be obtained in all cases. Care should be taken with intravenous drugs because of the risks of unrecognised fainting on induction, leading to hypotension and cerebrovascular insufficiency. Inhalational induction is well tolerated in children, particularly if sevoflurane is used and a good rapport is obtained between anaesthetist and patient. The standard method involves 30–40% oxygen in nitrous oxide with sevoflurane, introduced via a nasal mask. Once the mouth can be opened without resistance a pack is inserted by the surgeon which should separate the mouth from the pharynx. The teeth are then removed. The anaesthetist must maintain the airway, ensure oxygenation and anaesthesia and monitor the patient. Once the teeth have been removed and the mouth cleared of debris the inhaled anaesthetic agents are turned off and 100% oxygen administered until the child is awake. Recovery should be in the lateral ‘tonsillar’ position so that blood and debris are not inhaled. Debate has continued about whether the sitting or the supine position is better or safer. The supine position avoids unrecognised major falls in blood pressure but encourages regurgitation, whereas the sitting position discourages regurgitation but increases the likelihood of unrecognised fainting on induction.
Older children may accept dental extraction with local anaesthesia, but if extractions are proposed in more than two quadrants of the mouth then it is unwise to do this all at one sitting because of the inevitable risk of total anaesthesia of the tongue and palate leading to obstruction or aspiration.
General anaesthesia may be necessary for conservative dentistry in those with severe learning difficulties. This may be a prolonged procedure involving multiple restorations and should be carried out with the airway protected by either an endotracheal tube or a laryngeal mask and an absorbent pack in place. Dental drills have an incorporated water spray, which can precipitate laryngeal spasm in the unprotected airway.
ENT surgery
ENT surgery also requires a shared airway, typical procedures being tonsillectomy and adenoidectomy, where the surgeon is both operating in the airway and causing bleeding from it. Adenoidectomy in isolation requires the airway to be maintained via the mouth, either by tracheal tube or by laryngeal mask. Suction clearance of the mouth at the end of the procedure should be carried out under direct vision. Tonsillectomy in isolation may be carried out using either a nasotracheal tube or a laryngeal mask airway. The latter will require the surgeon’s compliance. Suction at the end must again be carried out under direct vision, but gently, so as not to disturb the tonsillar bed. The posterior nasal space in particular should be cleared of a potential ‘coroner’s clot’. In both of these cases postoperative analgesia should be provided parenterally before the recovery phase. There are advocates of both spontaneous and controlled ventilation for these procedures.
Anaesthesia for myringotomy or suction clearance of the ears can be relatively simple, using intravenous or inhalational induction with face mask or laryngeal mask for airway maintenance. Most patients are best recovered in the lateral position on a tipping trolley, or alternatively with a pillow under the thorax in the ‘tonsil’ position (Figure 5.14). Some patients will need to return to theatre in an emergency as a result of a post-tonsillectomy bleed (Figure 5.15).
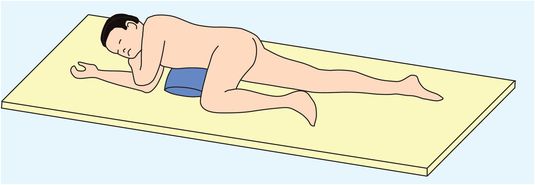
‘Tonsil’ position, with pillow under thorax
| Clinical problem | Blood in stomach, risk of aspiration Distress (child and parents) Hypovolaemic shock Pain |
| Immediate management | Airway – provide supplementary oxygen Breathing – tachypnoea is a bad sign Circulation – secure IV access, send FBC, cross-match blood Resuscitate as you go Call for help Prepare theatre and surgical team |
| Conduct of anaesthesia | Potential difficult airway (poor view due to bleeding) Need second experienced anaesthetist RSI versus inhalational induction Keep warm Continue resuscitation Recovery, assess volume status Recovery in tonsillar position Consider NG aspiration of gastric contents before waking Keep in theatre until completely happy (the ward will thank you!) |
Ophthalmic surgery
Ophthalmic surgery in the over-5s is usually for squint surgery or penetrating eye injury, probing and syringing of lachrymal ducts being confined to younger children.
Squint surgery is usually carried out as a day case, though the facility for overnight stay should always be available. Preoperative assessment should be as rigorous as for any other form of surgery. Induction of anaesthesia should include a weight-related dose of a vagolytic drug such as glycopyrrolate to prevent the severe bradycardia which results from even gentle traction on the extraocular muscles. Induction may be by the inhaled or intravenous route. Squint correction causes the same airway access problems for the anaesthetist as other head and neck surgery. The choice of tracheal intubation or laryngeal mask airway is largely a matter of personal preference, though tracheal tubes cause much more emergence laryngospasm than do laryngeal mask airways. Ventilation may be controlled or spontaneous. Postoperative analgesia may be provided by topical local anaesthetic agents, oral paracetamol or NSAIDs.
Child protection
Abuse takes many forms: physical, neglect, sexual and psychological (Figure 5.16). It also includes fabricated or induced illness. The anaesthetist may recognise non-accidental injury (NAI) because of his or her close involvement in the care of children from admission to discharge. NAI is not uncommon (about 7% in population), but sadly too often missed. Head trauma is the commonest presentation of abuse, particularly in young children, who may present in the resuscitation department or for a CT scan. The anaesthetist must maintain a high index of suspicion when there is an unusual presentation or unexplained elements in the history of injury, including abnormal/atypical clinical signs (e.g. atypical bruising patterns). In such circumstances the anaesthetist must always act in the best interests of the child.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







