Fig. 5.1
Examples of performance on clinical tests of neglect (patient’s performance is in red throughout). (a) Figure copying from the Behavioral Inattention Test [11] and clock drawing. (b) Line bisection performance with true center marked by the dashed line (note, this detail is absent in the actual test). (c) Two forms of cancellation task; star cancellation to the left and Albert’s lines to the right
A related disorder known as extinction is also assessed at the bedside [12, 13] by having patients fixate on the examiner’s nose while attending to the examiner’s left and right index fingers held out in the periphery. The patient must detect movement in the fingers with the examiner moving either the left or right finger alone (i.e., single stimulation) or both fingers together (i.e., double simultaneous stimulation). Patients with extinction can detect single events in left or right space, but “extinguish” the contralesional event in simultaneous stimulation trials. Neglect and extinction do co-occur; however, extinction can be evident in the absence of neglect and is equally common following left or right brain damage [12, 13]. Extinction and neglect also have distinct lesion foci [12, 14].
Although neglect is most prevalent for vision, it is commonly observed for auditory and tactile stimuli [5, 7, 8]. In addition, neglect is independent of low-level perceptual deficits and is best characterized not as a primary motor or perceptual deficit, but as a representational impairment. Perhaps the most famous demonstration of this comes from two patients tested by Bisiach and Luzzatti [15]. The patients were asked to imagine standing in a square in Milan and to report details they “saw” in their mind’s eye. When the patients were asked to imagine standing in the south, facing north, they reported details of the square from the right (east) and neglected details on the left (west). Conversely, when asked to imagine standing at the north, facing south, the patients now reported details from the previously neglected west and neglected details that they had initially reported from the east [15]. Similar demonstrations of what some have called “imaginal” neglect have been shown in other patients, including two with right hemisphere ischemic strokes [16, 17]. What this shows is that neglect patients have no deficit in recalling the details of mental representations. Instead, their mental representations are impoverished, containing only the right half of egocentric space.
As any clinician familiar with neglect can attest to, few patients fit the classic case. Neglect varies in terms of the reference frame within which symptoms predominate (e.g., personal versus extra-personal space) [18–23]. While most patients show neglect for stimuli defined strictly by spatial location, some show neglect for the left half of objects regardless of location [24, 25]. Finally, a plethora of “sub-syndromes” of neglect symptoms abound. For example, neglect dyslexia, in which patients fail to read the left half of words, is present in a handful of patients [26–28]. Although neglect was initially described some 60 years ago [29–31], the development of a conclusive theoretical account of the syndrome has proven elusive, in part due to the heterogeneity of symptoms.
Neglect as a Disorder of Spatial Attention
Traditional models of neglect stress the spatial nature of deficits [5–8, 32, 33]. In addition, given that neglect is more common after right-sided stroke or other brain damage, some suggest that the right parietal cortex is specialized for spatial attention [8, 34–37]. There are two component deficits to attention-based models of neglect: first, attention is captured strongly by right-sided stimuli. Second, patients have difficulty redirecting attention towards left space once attention is captured by right-sided stimuli. In other words, patients have difficulty disengaging attention from the right (i.e., a disengage deficit) [38, 39] (Fig. 5.2).
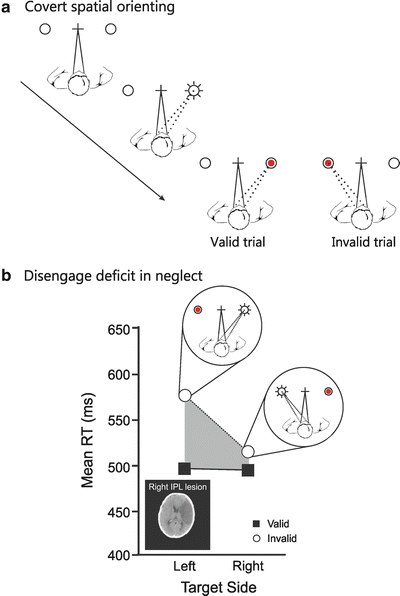

Fig. 5.2
(a) Schematic representation of trial sequences in the covert orienting of visual attention task [38, 39]. Patients fixate a central cross throughout while covertly attending to landmarks to the left and right. One landmark is cued (sun symbol in figure) drawing covert attention (dotted line) to that location. Targets can then appear at the cued (valid trial) or uncued (invalid trial) location with reaction times (RTs) faster on valid trials. (b) Performance of a neglect patient with a right inferior parietal lobule (IPL) lesion arising from a middle cerebral artery stroke. RTs are slowest to contralesional (left) targets appearing after an ipsilesional (right) cue (i.e., an invalid trial with the target appearing on the left)
Attentional capture for right-sided events can be seen in many perceptual biases in neglect. One such bias is evident on the chimeric faces task in which two vertically aligned chimerics (faces smiling on one side and neutral on the other) are shown with the patient indicating which appears to be happier. Controls choose the face shown as smiling on the left, reflective of right hemisphere dominance for emotional processing [40–42]. Neglect patients show a strong bias for choosing the face shown as smiling on the right [43]. Such biases are not unique to faces. When shown two rectangles that change in intensity from light to dark, patients choose the rectangle with the darkest end on the right as appearing darker—the opposite bias to that of controls [43]. Similar biases in intensity judgments can be seen for a broad range of stimuli, including numbers [44–47]. What these biases suggest is that for neglect patients, their attention is robustly captured by stimulus properties on the right [24]. Under some circumstances these biases actually confer a benefit to neglect patients, such that manual or saccadic reaction times are faster (relative to controls) for targets presented to restricted regions of right-sided space [48–51].
The second attentional impairment invoked in neglect models is the so-called disengage deficit. Best characterized as a difficulty in reorienting attention, Posner and colleagues [38, 39] showed that when patients with parietal lesions were cued to attend to ipsilesional space, their ability to detect contralesional targets was slowed (Fig. 5.2). Although evident after both left and right parietal damage and in patients with and without neglect, the impairment is most severe in neglect [52, 53]. In traditional models of neglect then, it is the combination of rightward attentional capture and a reorienting impairment (i.e., the disengage deficit) that determines the failure to respond to events in left space.
Deficits of Spatial Attention Fail to Capture the Full Syndrome of Neglect
It has become increasingly evident that attentional models of neglect do not capture the full gamut of symptoms evident in the syndrome. This has led to the suggestion that neglect is due to a constellation of symptoms that make it difficult for the patient to create and make use of a full and accurate representation of their surroundings [5]. Several of these components are discussed as follows.
Deficits in Nonspatial Sustained Attention
Robertson and colleagues [54, 55] first demonstrated sustained attention deficits in neglect using a simple task—the “Elevator Counting Task” [56, 57]. Patients were given between 3 and 14 tones separated by 3–5 s and had to report the number of tones presented. Neglect patients performed poorly on this task when compared with right brain-damaged patients without neglect [54]. Moreover, poor sustained attention correlated with neglect severity [54, 55]. The authors [54] suggest that poor sustained attention represents a critical marker of neglect and acts to exacerbate the more obvious spatial symptoms of the disorder [58–60]. Support for this notion comes from attempts to rehabilitate patients with neglect, which relies on modulating arousal levels [54, 55, 60, 61]. That is, when neglect patients are given brief loud tones intended to induce a phasic change to arousal, there is a concomitant improvement in some of the spatial symptoms of the disorder [61].
The ability to allocate attention over time is also impaired in neglect [62]. In the attentional blink task used to index this, patients attend to a stream of alpha-numeric stimuli and detect two targets embedded in the stream, with different temporal distances between the first and second targets [63, 64]. Controls show a diminished ability for discriminating target two when it is presented in close temporal proximity to target one (although there is spared capacity at the briefest lag) [65]. That is, once resources are allocated to target one, there is a refractory period—the attentional blink—during which those resources cannot be fully marshaled to discriminate target two. Neglect patients show an attentional blink almost 3 times longer than that observed in controls [62]. It should be noted that research shows an increased attentional blink with lesions of the left or right superior temporal gyrus (STG) [66], frontal cortex [67], and even cerebellum [68]. Given that impaired temporal allocation of attention is not unique to neglect, many suggest that this, and related deficits, reflect a disruption to tonic arousal levels that arise as a consequence of any neural insult. As such, impaired nonspatial sustained attention and poor temporal allocation of attention are thought to represent exacerbating symptoms that worsen the cardinal feature of neglect—an inability to consciously represent left space [59].
Spatial Working Memory
Two aspects of cancellation performance hint at problems with spatial working memory (SWM) in neglect that are not limited to left space. First, patients often fail to cancel targets in right space, suggesting they have a faulty representation of the spatial layout of the environment even for stimuli appearing in “good” or non-neglected space [5, 69]. Second, patients often place multiple cancellation marks on a single target, treating already processed or “old” items as if they are “new” (see Fig. 5.3). The tendency to treat old items as if they were new is especially evident when the patient’s cancellation marks are hidden from view [70, 71]. Conversely, revisiting behavior can be reduced when the salience of targets is reduced (or the target is removed) once cancelled by the patient [72].
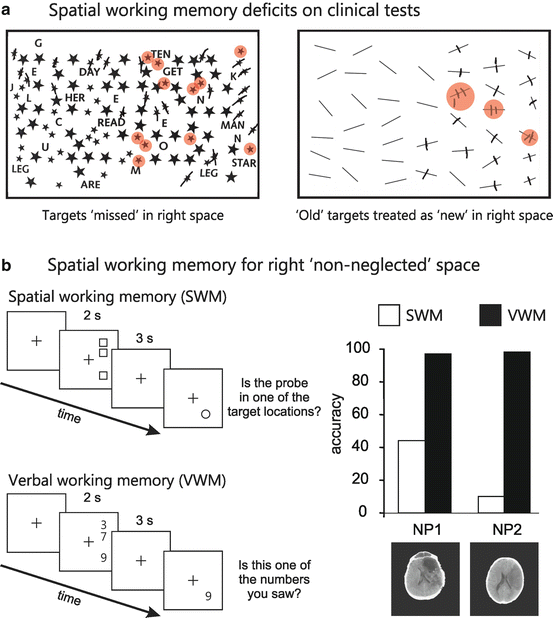

Fig. 5.3
(a) Spatial working memory (SWM) deficits on clinical tests of neglect—cancellation tasks. To the left is performance on the star cancellation task with targets missed on the right side of the array highlighted by red circles. To the right is performance on Albert’s lines task with targets that were revisited (i.e., “old” targets treated as if they were “new”) highlighted by red circles. (b) Performance of two neglect patients on a SWM and verbal working memory (VWM) task. For the SWM task, patients were presented with three vertically aligned targets and kept these locations in mind over a delay. A circle probe then appeared with the patient indicating whether the probe was presented in one of the previous target locations. For the VWM task patients were presented with three numbers to keep in mind over a delay and had to indicate whether a probe number presented after the delay was among one of the target group. To the right is accuracy (hits—false alarms) for both the SWM (white bars) and VWM (black bars) for two neglect patients. Adapted from [69]
Cancellation tasks are essentially a test of visual search performance, involve complex stimuli, and are reliant on multiple factors (e.g., perceptual discrimination, reorienting attention) to achieve good performance. Failure on such tasks could be due to impairments on any one or a combination of those factors. To address this, we (and others) have explored SWM in neglect [69, 73]. We asked patients to keep in mind over a short delay three vertically aligned targets. After the delay a probe appeared, with the patient indicating whether the probe appeared in one of the previous target locations (Fig. 5.3). A group of four neglect patients showed a severe deficit on this task even when the information to be remembered was presented to right, non-neglected, space [69]. This was in contrast to their normal ability to maintain alphanumeric stimuli over an identical delay [69]. This highlights that neglect patients do not have a generalized working memory deficit, but have a specific difficulty in maintaining spatial information over short periods of time. The fact that these deficits are not restricted to left, neglected space, but are instead evident for both central and right space [69, 73], makes it difficult to explain in the context of impaired allocation of spatial attention.
Impaired Perception of Time
Impoverished perception of time is another key deficit evident in neglect that defies explanation in terms of disrupted spatial or sustained attention [74–77]. Basso and colleagues [74] showed in one neglect patient that stimuli presented in the leftmost positions of an array (all items were presented in right, non-neglected space) were overestimated, whereas rightmost stimuli were underestimated. Harrington and colleagues [78] showed that patients with right frontoparietal lesions were unable to discriminate sub-second temporal durations. Finally, we showed that neglect patients dramatically underestimated multi-second durations [75]. Patients attended to an illusory motion stimulus for between 5 and 60 s and reported aloud the appearance of single digits presented at random temporal intervals (to prevent internal counting of durations). Interestingly, many patients failed to report several of these digits, mirroring the results of the aforementioned Elevator Counting and Attentional Blink tasks (although on different time scales). Each patient massively underestimated the elapsed time, never making estimates of greater than 10 s for what was actually a 60-s interval [75]. In another patient, we showed that this deficit was evident for visual, verbal, and nonverbal auditory stimuli, suggesting that impaired time perception is multimodal in neglect [76]. Finally, we tested two right medial temporal lobectomy patients on the visual time estimation task. Despite demonstrable memory deficits, these patients produced temporal estimates that were well within the range of healthy controls (Locklin and Danckert, unpublished data). This indicates that accurate time perception does not rely heavily on working memory processes, which were deficient in these patients (Fig. 5.4).
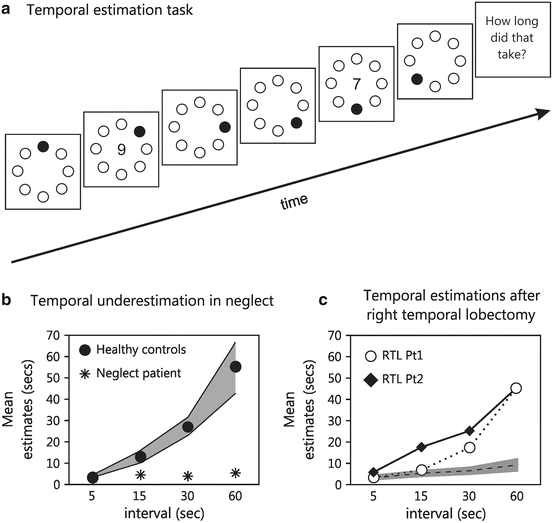

Fig. 5.4
(a) Schematic representation of the visual time estimation task. Patients saw an illusory motion stimulus for between 5 and 60 s and reported aloud numerals presented at random temporal intervals (to prevent internal counting of durations). (b) Mean estimates for healthy controls (black circles; SE represented by the gray bar) and one neglect patient (asterisk; see [75] for data from eight neglect patients and right brain-damaged patients without neglect). (c) Data from two patients who had undergone right temporal lobectomies (RTL) as treatment for medication resistant epilepsy. Both patients show adequate temporal estimations despite substantial working memory deficiencies indicating that for neglect patients, disordered time estimation is unlikely to be related to memory capacity
An inability to accurately represent time in and of itself cannot explain, nor be explained by the spatial nature of neglect. What this kind of deficit represents is a difficulty in accounting for a key component of an ever-changing environment—the temporal dynamics of those changes. For a neglect patient who is already biased to attend only to right space, an inability to time stamp changes to incoming information will compound this deficit, making it more difficult to represent changes in the environment, particularly when they occur in left space.
Representational Updating in Neglect
As discussed earlier, neglect has been considered a disorder of mental representations [15]. In other words, neglect represents an inability to create or appropriately make use of mental models of the environment. Many of the spatial and nonspatial deficits (or at the very least non-spatially lateralized deficits—cf. SWM) in neglect can be parsimoniously characterized as deficits in mental model building and updating. Mental models have been shown to subserve a wide variety of functions including inferring intention (i.e., theory of mind) [79], predicting the sensory consequences of actions (a capacity impaired after parietal damage) [80–83], and learning new skills based on prior experiences [84].
Recent theories have suggested that neglect is best characterized as a combination of deficits in spatial attention and difficulties in remapping space [85]. The task used to test spatial remapping is known as the double-step saccade task, in which two targets for successive eye movements are presented in rapid succession [86]. Programming both saccades based on retinal signals leads to an erroneous saccade to target two. Instead, we remap our model of space based on the anticipated outcome of the saccade to target one. Spatial remapping is clearly deficient after parietal lobe injury, due in the cited cases to aneurysmal subarachnoid hemorrhage, ischemic stroke, and brain tumor surgery [86, 87]. Nevertheless, deficits in spatial remapping still fail to account for many of the symptoms discussed previously including poor SWM, which is not restricted to any particular region of space and impaired perception of time [69, 75, 76]. In addition, recent work employing prisms to rehabilitate neglect (see section “Recovery from Neglect”) has shown that while some spatial symptoms can be improved, many other aspects, including perceptual biases, SWM, and time perception deficits, remain unchanged [88, 89]. This suggests there are dissociable components to the neglect syndrome, only some of which are ameliorated via attentional training.
One of the challenges in theorizing about neglect arises due to the heterogeneity of symptom profiles [5, 32]. What is suggested here is that neglect likely arises as a consequence of a combination of key impairments including poor spatial orienting and sustained attention and what could be characterized as a generalized impairment in updating mental models. With respect to the latter, deficits in saccadic remapping, SWM, motor imagery, time perception, and to some degree even spatial attention [90] are recast as impairments to the ability to generate and make use of mental models of the environment [91, 92].
Mental models represent learned rules and expectations concerning the way in which the world operates and enable us to simplify the processing needed to control behavior in flexible and optimal ways. Necessarily, these models require frequent updating as new information indicates a change in environmental state. Such updating requires some form of comparison to determine whether new information matches the rules and expectations of our model. When mismatches arise, the degree or type of mismatch determines whether the model needs updating. This kind of comparator process has been invoked to describe the role of parietal cortex in motor control and motor imagery [83, 93–95]. What is being proposed here is that a more generic kind of mental model updating process could be ascribed to the inferior parietal lobule that, when damaged, would explain many of the key symptoms of neglect—symptoms that defy a simple attentional explanation. If the inferior parietal lobule is found to support the generation and updating of mental models, one would expect to see updating deficits evident not only in spatial (e.g., impaired saccadic remapping, SWM) domains, but also in nonspatial domains.
We examined whether neglect patients would show a deficit in updating mental models in a nonspatial domain by having them play the children’s game “rock, paper, scissors” (Fig. 5.5) [96].
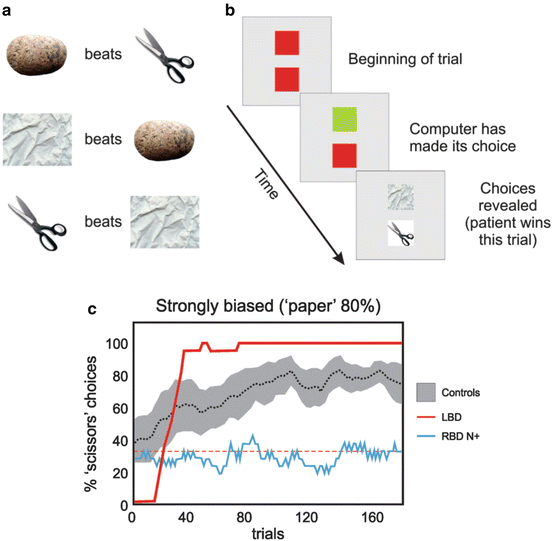

Fig. 5.5
(a) Representation of the rules governing the zero-sum children’s game “rock, paper, scissors.” (b) Schematic representation of the version of “rock, paper, scissors” played with neglect patients in [96]. The top square represents the computer’s play and this changed from red to green when the computer had “locked in” its choice. Patients then made their choices and both were revealed to indicate the outcome for the patient (win, loss, tie; in the instance shown here, the patient wins). (c) Performance for the condition in which the computer adopted a strongly biased play strategy (i.e., choosing “paper” on 80 % of all trials). Participant choices are represented as a moving average (n = 20 trials) of the optimal choice (“scissors”). Controls (dotted line, gray bar represents standard error) quickly discern the computer’s strategy and adopt the optimal choice around 75 % of the time (i.e., matching the computer’s play). A typical left brain-damaged (LBD) patient is shown in red. This patient quickly chose to exploit the biased play of the computer. Unlike controls, the patient maximized their response choices (i.e., choosing “scissors” 100 % of the time). This was a common strategy seen in 9 of 10 LBD patients. A typical right brain-damaged patient with neglect (RBD N+) is shown in blue. Clearly, this patient’s play did not deviate from random, a pattern that was observed in 5 of 7 neglect patients [96]
Their computer opponent initially played a uniform strategy choosing one option on 33 % of trials (no strategy the patient could adopt will lead to more than 33 % wins). Later, the computer adopted a biased strategy choosing one option (“paper”) on 80 % of trials. The logic here is that an accurate model of your opponent’s play will allow you to adjust your own strategy to exploit that bias and maximize wins (e.g., choose “scissors” most often). Five of seven neglect patients failed to take advantage of the biased play of the computer opponent (Fig. 5.5) [96]. The two who did alter their play took more than 3 times as long as controls to do so and never reached the optimal levels of controls. We also observed impaired performance in some right brain-damaged patients without neglect, indicating that an updating deficit is not unique to neglect. This is an all too common refrain in the neglect literature with even what is sometimes considered to be a cardinal deficit (i.e., impaired orienting to left space) showing up in right brain-damaged patients without neglect (note: the same is true for saccadic remapping and temporal deficits, although impairments are consistently worst for neglect patients) [53, 66, 75, 87]. What this indicates is that no single deficit alone is sufficient to produce the full neglect syndrome. Instead, neglect arises as a consequence of a constellation of key impairments, one of which is the inability to generate or update mental models of the environment across multiple domains and behaviors.
What the preceding sections emphasize is that a neglect patient already captured by events in right space will experience great difficulty exploring the world beyond that region, given that his/her internal model of the environment either is poorly constructed in the first instance or does not undergo appropriate updating relevant to changes in the environment in the second instance.
Anatomy of Neglect
As already mentioned, neglect is a heterogeneous syndrome. The precise array of deficits evident in any patient will vary with lesion location and the extent to which white matter pathways are involved. This section outlines some of the recent work exploring the critical lesion site for neglect.
What Is the Critical Lesion for Neglect?
The earliest descriptions of neglect implicated damage to the supramarginal and angular gyri [30, 31]. Many early studies did not have the benefit of imaging (although Paterson and Zangwill [30] did make use of X-rays) and often relied on autopsy, where available, to determine structure-function relationships. The first study to attempt to determine the common region involved in neglect used computed tomography (CT) scans and suggested the inferior parietal cortices were most commonly involved [97]. More recently some controversy has arisen concerning the critical lesion for neglect (Table 5.1) [98, 99]. These studies variously claim that the STG or the angular gyrus of the inferior parietal lobule (IPL) represent the critical lesion site for neglect (Table 5.1 and Fig. 5.6) [98, 99].
Table 5.1
Summary of studies exploring the anatomical basis of neglect
References | # Patients | Method | Critical region associated with neglect |
|---|---|---|---|
Buxbaum et al. [101] | 166 (80 neglect, 42 acute, 38 chronic) | CT/MRI | Acute = BG, inf/mesial temp |
Chronic = cingulate, OFC, IPL, IT, inf/mesial temp, occ, sup/mid temp | |||
Chechlacz et al. [102] | 41 (21 RH patients; 19 left neglect, 4 right neglect) | DTI, VLSM | IPS, TPJ, SLF, ILF, SFOF, IFOF, thalamic radiation, corona radiata |
Allocentric neglect = pSTS, AG, mid temp, mid occ | |||
Egocentric neglect = mid front, PCG, STG, SMG | |||
Karnath et al. [98] | 33 neglect patients (25 w/o field cuts) | MRI, VLSM | STG, ventral PCG, parietal operculum |
140 RH (78 neglect) | MRI, VLSM | STG, insula, putamen, caudate, SLF, IFOF, SFOF, | |
Karnath et al. [134] | 54 (24 neglect; 8 chronic, 16 recovered) | MRI, VLSM | STG, MTG, BG, IFOF, extreme capsule + uncinate fasciculus in chronic patients |
Mort et al. [99] | 35 (24 MCA/14 neglect; 11 PCA/5 neglect) | MRI | MCA = AG, STG in 50 % |
PCA = Parahippocampal | |||
Ptak and Schnider [135] | 30 RBD (20 neglect) | VLSM | TPJ, pIPS, MFG |
Urbanski et al. [111] | 4 (2 neglect) | DTI | IPL, IFOF |
Urbanski et al. [110] | 12 (6 neglect) | DTI | Perisylvian w.m., ant. limb of IC, w.m. underlying IFG (ant. segment of arcuate fasciculus) |
Vallar and Perani [97] | 110 (47 neglect, 29 severe neglect) | CT | IPL, thalamus, BG |
Verdon et al. [104] | 80 (55 neglect, 16 severe neglect) | MRI, VLSM | Visuospatial neglect = IPL |
Motor neglect = DLPFC | |||
Allocentric neglect = parahippocampal gyrus |








