Gram + aerobic
Gram − aerobic
Anaerobic
Marine vibrio
Fungi
Group A streptococcus
Escherichia coli (cirrhosis)
Bacteroides sp. (DM)
Vibrio vulnificus (cirrhosis)
Candida (immunosuppression)
Group B Streptococcus (DM)
Pseudomonas aeruginosa sp. (immunosuppression and burns)
Clostridium sp. (Intravenous drug use, colonic neoplasms, contaminated wounds)
Vibrio parahaemolyticus (cirrhosis)
Aspergillus (immunosuppression)
Staphylococcus aureus (DM)
Enterobacter cloacae
Peptostreptococcus sp
Vibrio damsela
Zygmocetes (immunosuppression)
Coagulase negative staphylococci
Klebsiella sp. (cirrhosis and DM)
Peptococcus sp.
Vibrio alginolyticus (cirrhosis)
Enterococci (DM)
Proteus sp.
Bacilli
Seratia sp. (chronic renal failure and DM)
Acinetobacter
Citrobacter freundii
Pasteurella multocida (intravenous drug use)
Epidemiology
Multiple intraabdominal conditions may lead to NSTI. Perforated appendicitis, colon cancer, diverticulitis, inflammatory bowel disease and complex, neglected or inadequately treated perianal abscesses are the leading etiologies related to colon and rectal pathology. Additionally, gastroduodenal or small bowel perforations and gangrenous cholecystitis are other potential intraabdominal causes of NSTI. NSTI can also follow elective or emergency gynecologic, genitourinary, abdominal, or rectal surgical procedures. The abdominal wall is the site of NSTI in approximately 5–10 % [1] infections.
Pathophysiology
In general, necrotizing infections result from bacterial invasion into the subcutaneous fat or superficial fascia. Enzymes and toxins produced by the bacteria facilitate the spread along the fascial planes deep below the skin surface [2]. Bacterial invasion produces tissue necrosis by causing vascular thrombosis leading to tissue death. This environment favors the growth of anaerobic or facultative anaerobic bacteria. The hallmark of NSTI is the accumulation of gas formed by these bacteria. A variety of gases are produced in the low-oxidative reduction potential environment including hydrogen, nitrogen, hydrogen sulfide, and methane which then accumulate in the soft tissue spaces [3]. Perforation of the retroperitoneal colon or pelvic portion of the rectum may introduce air and bacteria into the retroperitoneum or peritoneum. Abscesses may subsequently develop in these spaces and extend along fascial pains or necessitate to the skin surface.
Anatomy
The site of perforation in the bowel will govern the route of spread to the subcutaneous position. Intraperitoneal perforations dissect through defects in the peritoneum or mesenteric attachments. A violation of the peritoneum or fascia neighboring the injury leads to the intramuscular and subcutaneous surfaces. The infection can spread contiguously to extend from the retroperitoneum to the deep abdominal fascia and ultimately through the abdominal skin or exit through the obturator foramen to spread to the gluteal region or the thigh (see Fig. 18.1). This can lead to involvement of the limb by following the iliacus or psoas muscle to their insertions on the greater and lesser trochanters, respectively (see Figs. 18.1 and 18.2).
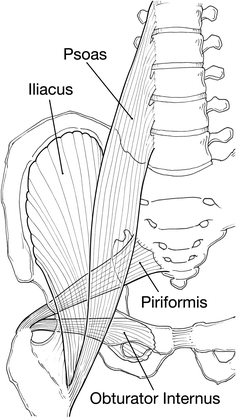
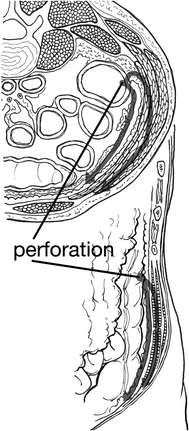

Fig. 18.1
Four major pelvic muscles and their extremity attachements, Psoas major origin transverse processes of lumbar spine inserts on lesser trochanter, Iliacus origin upper portion of the iliac fossa and sacrum and inserts on lesser trocanter, Pyriformis origin sacrum and sciatic foramen and inserts on greater trochanter, Obturator internus origin obturator foramen margins and inserts on lesser trochanter

Fig. 18.2
Demonstrates path of bacterial spread from colon perforation with relationship to abdominal wall
Solid organs or intact fibrous membranes offer good resistance to extension while areolar and loose fascial tissues offer significantly lower resistance. Muscular insertions and vascular investing fascia may conduct the spread of intrapelvic and retroperitoneal infection to the buttocks, hip, thighs, and perineum (see Figs. 18.1 and 18.3). Studies using cadavers show that air injected into the posterior peritoneum produced air in the anterior peritoneal area while air injected into the presacral space produced air in the lower abdominal wall, thighs, scrotum, and buttocks [4]. The routes of spread include passage along neurovascular bundles that penetrate muscle and abdominal wall fascia, through natural defects such as the inguinal ring, along the femoral vessels and over the inguinal ligaments and through the pelvic floor to the tissues of the buttocks and thighs and over the abdomen [5]. Air present in the soft tissues may represent dissected air from the aerodigestive tract or air produced by infecting organisms. Perforation of the bowel should be excluded when subcutaneous emphysema of the abdominal wall is identified.
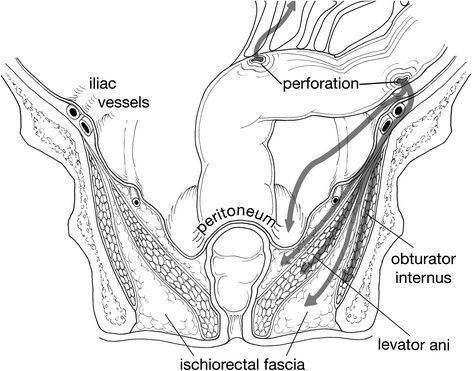

Fig. 18.3
Note endopelvic fascia covers major pelvic muscles, Arrows demonstrate path of potential bacterial spread from colonic perforations
Bacteriology
NSTI related to perforations are overwhelmingly polymicrobial reflecting the microbiota of the colon. However, patient factors may alter host defenses favoring the growth of particular organisms. See Table 18.1. Antibiotics should be directed against likely pathogens including anaerobic organisms. Fungal infection is possible with perforated bowel especially with nosocomial infections or an immunocompromised host. See Table 18.2 for microbes and effective anti-infective therapy.
Table 18.2
Microorganisms associated with necrotizing fasciitis and antibiotic therapy
Microbe | First line therapy | Penicillin allergy |
|---|---|---|
Mixed infections | Piperacillin-tazobactam and vancomycin Imipenem-cilastatin Meropenem Ertapenem Cefotaxime and Metronidazole or clindamycin | Clindamycin or metronidazole with an aminoglycoside or fluroquinolone |
Streptococcus | Penicillin plus clindamycin | Vancomycin, linezolid, quinupristin/dalfopristin, daptomycin |
Staphylococcus aureus (SA) | Nafcillin Oxacillin Cefazolin Vancomycin (for resistance) Clindamycin | Vancomycin, linezolid, quinupristin/dalfopristin, daptomycin Bacteriostatic; inducible resistance in methicillin resistant SA |
Clostridial species | Clindamycin plus penicillin | |
Aeromonas hydrophila | Doxycycline plus ciprofloxacin or ceftriaxone | |
Vibrio Vulnificus | Doxycycline plus ciprofloxacin or ceftriaxone | |
Candida albicans | Caspafungin, micafungin or fluconazole | |
Aspergillus | Voriconazole, lipid formulation amphotericin B |
Presentation
Hard signs of necrotizing fasciitis are classically tense edema, violaceous bullae, and crepitance. When necrotizing fasciitis results from bowel perforation, it is usually because of a delay in the recognition of the problem or presentation of the patient. Intraperitoneal colonic perforation usually presents overtly with signs of peritonitis, fever, and tachycardia. Abdominal-wall skin changes and crepitus are not a part of the usual presentation. A retroperitoneal perforation may be at least partially walled off and sequestered from the peritoneum. This would limit the peritoneal irritation and subsequent discomfort that would be anticipated. Further extension into the flank musculature and fascia may produce pain that can be confused with an extra abdominal source confounding the diagnosis. As the infection spreads and tissue destruction progresses, skin changes may become evident. The most common signs include tenderness (often out of proportion to physical findings), erythema, and induration. Unfortunately, these findings are rather nonspecific and are seen with non-necrotizing soft tissue infections and trauma. Alternatively, depending on the cause of the perforation, gastrointestinal symptoms may be more prominent and influence the diagnostic evaluation or lead to urgent laparotomy. Nausea, vomiting, diarrhea, constipation or obstipation, melena, hematochezia, and weight loss are evidence of a likely intraabdominal problem. When these symptoms coincide with the presence of abdominal wall, flank or even limb skin changes or crepitus, abdominal imaging to identify the source of pathology will be useful. Patients with NSTI related to bowel perforation present with signs related to the bowel perforation or to the soft tissue infection or with some combination of the two (see Table 18.3).
Table 18.3
Signs and symptoms associated with necrotizing fasciitis
General | Frequency (%) | Systemic | Frequency (%) | GI related |
|---|---|---|---|---|
Erythema | 70 | Fever | 40 | Nausea Vomiting Constipation Diarrhea Abdominal pain Hematochezia Melena Bloating Anorexia Draining (fecalent) sinus |
Warmth | 44 | Hypotension | 21 | |
Tachypnea (>20) | 26 | |||
Tenderness | 79 | + Blood culture | 35 | |
Induration | 66 | Mental status changes | 5 | |
Bullae/blisters | 25 | Tachycardia | 59 | |
Creptius | 20 | Tachypnea | 26 | |
Skin necrosis | 24 | |||
Swelling | 80 | |||
Drainage | 19 |
There are a variety of patient factors that effect the clinical presentation. Patients with altered pain perception are a group at high risk for delayed presentation or diagnosis. Both patients and providers easily ignore early subtle findings. This group includes patients who abuse narcotics and alcohol. They may self-medicate thereby masking their symptoms or their symptoms may be overlooked when they do seek medical care. As a result, some of these patients have obvious skin necrosis and/or are in septic shock when they present.
The host’s ability to contain the bacteria can impact the development of NSTI. Immunosuppressed patients are at high risk for a delay in diagnosis of perforation [6]. Without the normal inflammatory response to injury, these patients lack a classical presentation. Fever, erythema, and pain may be mild or absent.
Diabetes Mellitus and morbid obesity can interfere with the presentation of signs and symptoms. Many diabetic patients have neuropathy that may alter their pain perception, and poor glycemic control certainly alters immune function. Abdominal evaluation in the morbidly obese patient is notoriously difficult. Elderly patients may also present with atypical symptoms or blunted responses making prompt diagnosis difficult (see Table 18.4 for a list of associated medical conditions).
Table 18.4
Associated medical conditions
Associated medical condition |
|---|
Diabetes |
Advanced age |
Cirrhosis |
Renal failure |
Immunosupression |
Peripheral vascular disease |
Intravenous drug use |
Alcohol abuse |
Obesity |
Investigation
In addition to a comprehensive physical examination, laboratory tests may help to confirm or raise the level of concern for NSTI. As is true of the physical examination, many routine laboratory tests results are nonspecific, but in combination with physical findings, laboratory abnormalities can improve diagnostic precision [7–9] (see Table 18.5). Serum carcinoembryonic antigen (CEA) should be measured when perforated colon carcinoma is suspected. The patient that presents in septic shock with “hard signs” of NSTI does not usually require further diagnostic investigation. Laboratory studies are used to gauge the degree of shock and organ dysfunction and to monitor resuscitation.
Table 18.5
Useful laboratory studies
Blood test | Value | Laboratory risk necrotizing fasciitis indicator score (LRINEC) >6 | |
|---|---|---|---|
WBC per mm3 | >15 | <15 | 0 |
15–25 | +1 | ||
>25 | +2 | ||
Na meq/dL | <135 | >135 | 0 |
<135 | +2 | ||
Glucose mg/dL | >180 | <180 | 0 |
>180 | +1 | ||
Creatinine mg/dL | >1.6 | <1.6 | 0 |
>1.6 | +2 | ||
C reactive protein mg/dL | >15
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||





