KEY POINTS
Shock is present when there is evidence of multisystem organ hypoperfusion; it often presents as decreased mean blood pressure.
Initial resuscitation aims to establish adequate airway, breathing, and circulation. Rapid initial resuscitation (usefully driven by protocol) is fundamental for improved outcome, since “time is tissue.”
A working diagnosis or clinical hypothesis of the cause of shock should always be made immediately, while treatment is initiated, based on clinical presentation, physical examination, and by observing the response to therapy.
Drug and/or definitive therapy for specific causes of shock must be considered and implemented early (eg, hemostasis for hemorrhage, revascularization for myocardial infarction, appropriate antibiotics, etc).
The most common causes of shock are high cardiac output hypotension, or septic shock; reduced venous return despite normal pump function, or hypovolemic shock; reduced pump function of the heart, or cardiogenic shock; and obstruction of the circulation, or obstructive shock. Overlapping etiologies can confuse the diagnosis, as can a short list of other less common etiologies, which are often separated by echocardiography and pulmonary artery catheterization.
Shock has a hemodynamic component, which is the focus of the initial resuscitation, but shock has also a systemic inflammatory component (ameliorated by rapid initial resuscitation) that leads to adverse sequelae including subsequent organ system dysfunction.
This chapter discusses shock with respect to the bedside approach: first with an early working diagnosis, then an approach to urgent resuscitation that confirms or changes the working diagnosis, followed by a pause to ponder the broader differential diagnosis of the types of shock and the pathophysiology of shock leading to potential adverse sequelae. Effective initial diagnosis and treatment at a rapid pace depend in large part on understanding cardiovascular pathophysiology.
ESTABLISHING A WORKING DIAGNOSIS OF THE CAUSE OF SHOCK
Shock is present if evidence of multisystem organ hypoperfusion is apparent. Evidence of hypoperfusion includes tachycardia, tachypnea, low mean blood pressure, diaphoresis, poorly perfused skin and extremities, altered mental status, and decreased urine output. Hypotension has special importance because it commonly occurs during shock, because blood pressure is easily measured, and because extreme hypotension always results in shock. Important caveats are (1) relatively low blood pressure is normal in some healthy individuals and (2) systolic blood pressure may be preserved in some patients in shock by excessive sympathetic tone. In the latter case, it is important to anticipate that sedation will unmask hypotension. Further, cuff blood pressure measurements may markedly underestimate central blood pressure in low flow states.1 The focus of initial resuscitation is reversing the hemodynamic component of shock, which leads to tissue hypoxia and lactic acidosis. However, all types of shock are also associated with a systemic inflammatory component that is a key contributor to subsequent multisystem organ failure and death. The development of the systemic inflammatory component is minimized by rapid and adequate (usefully driven by protocol) initial resuscitation.2,3
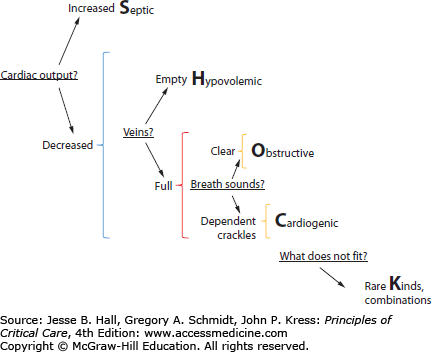
Mean blood pressure is the product of cardiac output and systemic vascular resistance (SVR). Accordingly, hypotension may be caused by reduced cardiac output or reduced SVR. Therefore, initial examination of the hypotensive patient seeks to answer the question: Is cardiac output increased or decreased? (Fig. 33-1) High cardiac output hypotension is most often signaled by a high pulse pressure, a low diastolic pressure, warm extremities with good nail bed return, fever (or hypothermia), leukocytosis (or leukopenia), and other evidence of infection; these clinical findings strongly suggest a working diagnosis of septic shock (Table 33-1), the initial treatment for which is thoughtful antibiosis combined with rapid expansion of the vascular volume and subsequent vasopressors, inotropes, and blood transfusion as necessary to achieve an adequate central venous pressure (CVP), mean arterial pressure (MAP), and central venous oxygen saturation (ScvO2; see below).
FIGURE 33-1
The different types of shock can be remembered using the SHOCK mnemonic and defined by asking four questions. First, is cardiac output increased (Septic shock) or decreased (other forms)? Second, are central veins empty (Hypovolemic shock) or full (other forms)? Third, are breath sounds clear (Obstructive shock) or are crackles heard (Cardiogenic shock)? Finally, what does not fit? This identifies combinations (eg, septic shock with hypovolemia) or rare kinds of shock. Additional physical findings, laboratory tests, and echocardiographic and other examinations illuminate these simplified questions further.
Rapid Formulation of an Early Working Diagnosis of the Etiology of Shock
| Defining features of shock | ||
| Blood pressure | ↓ | |
| Heart rate | ↑ | |
| Respiratory rate | ↑ | |
| Mentation | ↓ | |
| Urine output | ↓ | |
| Arterial pH | ↓ | |
| High-Output Hypotension: Septic Shock | Low Cardiac Output Shock: Cardiogenic and Hypovolemic Shock | |
| Is cardiac output reduced? | No | Yes |
| Pulse pressure | ↑ | ↓ |
| Diastolic pressure | ↓ | ↓ |
| Extremities/digits | Warm | Cool |
| Nail bed return | Rapid | Slow |
| Heart sounds | Crisp | Muffled |
| Temperature | ↑ or ↓ | ↔ |
| White cell count | ↑ or ↓ | ↔ |
| Site of infection | ++ | — |
| Reduced pump function, cardiogenic shock | Reduced venous return, hypovolemic shock | |
| Is the heart too full? | Yes | No |
| Symptoms, clinical context | Angina, abnormal electrocardiogram | Hemorrhage, dehydration |
| Jugular venous pressure | ↑ | ↓ |
| S3, S4, gallop rhythm | +++ | — |
| Respiratory crepitations | +++ | — |
| Chest radiograph | Large heart | Normal |
| ↑ Upper lobe flow Pulmonary edema | ||
| What does not fit? | ||
| Overlapping etiologies (septic cardiogenic, septic hypovolemic, cardiogenic hypovolemic) | ||
| Short list of other etiologies | ||
High-output hypotension Liver failure Severe pancreatitis Trauma with significant systemic inflammatory response Thyroid storm Arteriovenous fistula Paget disease Get more information Echocardiography, right heart catheterization | High right atrial pressure hypotension Pulmonary hypertension (Most often pulmonary embolus) Right ventricular infarction Cardiac tamponade | Nonresponsive hypovolemia Adrenal insufficiency Anaphylaxis Spinal shock |
In contrast, low cardiac output is signaled by a low pulse pressure, mottled cyanotic skin, and cool extremities with poor nail bed return. In this case, clinical examination turns to a second question: Are central veins empty or full? (Fig. 33-1) When low cardiac output results from hypovolemia (see Table 33-1) clinical examination shows manifestations of blood loss (hematemesis, tarry stools, abdominal distention, reduced hematocrit, or trauma) or manifestations of dehydration (reduced tissue turgor, vomiting or diarrhea, or negative fluid balance). In contrast, elevated jugular veins in a hypotensive patient suggest either obstruction (eg, pulmonary embolism, cardiac tamponade) or cardiogenic shock (Fig. 33-1) raising the third question: Are breath sounds normal? Cardiogenic shock is distinguished from obstructive shock by dependent crackles on lung auscultation, a laterally displaced precordial apical impulse with extra heart sounds (S3, S4), peripheral edema, chest pain, ischemic changes on the electrocardiogram, and a chest radiograph showing a large heart with dilated upper lobe vessels and pulmonary edema.4
Whenever the clinical formulation is not obvious after answering the first three questions, ask a fourth: What does not fit? Most often, the answer is that the hypotension is due to overlap of two or more of these common etiologies of shock: septic shock complicated by hypovolemia or myocardial dysfunction, cardiogenic shock complicated by hypovolemia or sepsis, etc. At this time, more data are frequently needed, especially aided by echocardiography. Interpretations of the data and response to initial therapy frequently confirm the multiple etiologies or lead to a broader differential diagnosis of the etiologies of shock (see below). A short list of common etiologies other than septic, hypovolemic, obstructive, or cardiogenic shock can be grouped as they present (see Table 33-1): high cardiac output hypotension that does not appear to be caused by sepsis and poorly responsive hypovolemic shock.
URGENT INITIAL RESUSCITATION
Early institution of aggressive resuscitation improves a patient’s chances of survival.2,3 To improve efficiency at the necessarily rapid tempo, a systematic approach to initial evaluation and resuscitation is useful as it is during cardiac emergencies (advanced cardiac life support [ACLS]) and trauma (advanced trauma life support [ATLS]). In analogy to these systematic “ABC” approaches, a primary survey includes establishing an airway (airway), choosing a ventilator mode and small tidal volumes that minimize ventilator-induced lung injury (breathing), rapid (usefully protocol driven) resuscitation of the inadequate circulation (circulation), and drugs/definitive therapy consisting of early consideration and implementation of definitive therapy for specific causes of shock (eg, hemostasis for hemorrhage, revascularization for myocardial infarction, appropriate antibiotics, surgical drainage of abscess, etc).
Airway: Almost all patients in shock have one or more indications for airway intubation and mechanical ventilation (Table 33-2), which should be instituted early. Significant hypoxemia (based on blood-gas analysis, pulse oximetry, or high clinical suspicion) is one indication for airway intubation because external masks and other devices do not reliably deliver an adequate fraction of inspired O2 (FiO2). Initially, a high FiO2 (100%) is used until blood-gas analysis or reliable pulse oximetry allows titration of the FiO2 down toward less toxic concentrations.
Indications for Intubation in Shock Patients
| Indication | Why |
|---|---|
| Hypoxemia | High FiO2 is not guaranteed by oxygen masks; PEEP can be added |
| Ventilatory failure (inappropriately high PCO2, signs of ventilatory muscle fatigue) | Ensure adequate CO2 removal |
| Correct hypoxia due to hypoventilation | |
| Prevent sudden respiratory arrest | |
| Vital organ hypoperfusion | Rest ventilatory muscles (and divert cardiac output to hypoperfused vital organs) |
| Obtundation | Protect and ensure an adequate airway |
Ventilatory failure is another indication for airway intubation and mechanical ventilation. Elevated and rising partial pressure of CO2 in arterial blood reliably establishes the diagnosis of ventilatory failure but is often a late finding. In particular, young, previously healthy patients are able to defend partial pressure of CO2 (PCO2) and pH up until a precipitous respiratory arrest. Therefore, clinical signs of respiratory muscle fatigue or subtle evidence of inadequate ventilation are more important early indicators.5 Evidence of respiratory muscle fatigue, including labored breathing precluding more than rudimentary verbal responses, tachypnea greater than 40/min or an inappropriately low and decreasing respiratory rate, abdominal paradoxical respiratory motion, accessory muscle use, and other manifestations of ventilatory failure such as inadequately compensated acidemia should lead to early elective intubation and ventilation of the patient in shock (see Chap. 43).
Obtundation, due to shock or other causes, resulting in inadequate airway protection is an important indication for intubation. In shock, airway intubation and mechanical ventilation should precede other complicated procedures, such as central venous catheterization, or complicated tests that require transportation of the patient when these procedures and tests restrict the medical staff’s ability to continuously assess the airway and ensure adequacy of ventilation.
Breathing: Initially, mechanical ventilation with sedation and, if necessary, paralysis are instituted to remove work of breathing as a confounding factor from the initial resuscitation and diagnostic pathway and to redistribute limited blood flow to vital organs.6 The change from spontaneous breathing (negative intrathoracic pressure ventilation) to mechanical ventilation (positive intrathoracic pressure ventilation) leads to reduced venous return so that additional volume resuscitation must be anticipated when hypovolemia contributes to shock. Application of positive end-expiratory pressure (increases intrathoracic pressure) and administration of sedative or narcotic drugs (increases venous capacitance) similarly should be expected to reduce venous return and highlight the importance of aggressive volume resuscitation at the time of intubation and institution of mechanical ventilation in hypovolemic patients. Conversely, when hypovolemia is not a problem (eg, cardiogenic shock), application of positive intrathoracic pressure may improve cardiac output and blood pressure.
A relatively small tidal volume (6-8 mL/kg) should be selected to minimize hypotension due to high intrathoracic pressures and, more importantly, to reduce ventilator-induced lung injury.7 When arterial hypoxemia due to acute respiratory distress syndrome (ARDS) complicates shock, adherence to tidal volumes of 6 mL/kg ideal body weight significantly decreases mortality rate and number of days on a ventilator in the intensive care unit.8
Circulation
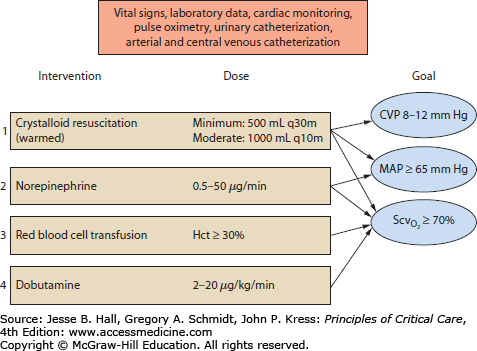
Goals and Monitoring Just as low tidal volumes limit ongoing lung inflammation and injury, rapid resuscitation of the circulation limits ongoing generation of a systemic inflammatory response and multiple organ injury. Hence, rapid protocol-driven approaches with defined end points improve shock outcome.2,3 For all types of shock, “time is tissue.” Thus, for hypovolemic shock due to hemorrhage, the early goal is immediate hemostasis and rapid volume resuscitation. For cardiogenic shock secondary to acute myocardial infarction, the early goal is immediate thrombolysis, angioplasty, or surgical revascularization.9 For obstructive shock, relief of tamponade, lysis or removal of the massive pulmonary embolus, or surgical relief of abdominal compartment syndrome is required. The early goals of volume resuscitation in hypovolemic or septic shock are incorporated in the Early Goal-Directed Therapy algorithm (Fig. 33-2), which was initially designed to aid resuscitation of septic shock.3 This requires immediate monitoring (even before formal admission to the intensive care unit) of central venous pressure (CVP; goal 8-2 mm Hg), MAP (goal >65 mm Hg), and ScvO2 (goal >70%). When ScvO2 is not readily measured then lactate clearance of >10% over ∼2 hours is a reasonable alternative goal.10
FIGURE 33-2
An approach to initial resuscitation of the circulation based on Early Goal-Directed Therapy. Cardiac monitoring, pulse oximetry, urinary catheterization, and arterial and central venous catheterizations must be instituted. Volume resuscitation is the initial step. If this is insufficient to raise mean arterial pressure (MAP) to 65 mm Hg, then vasopressors are the second or simultaneous step. Adequate tissue oxygenation (reflected by central venous O2 saturation [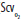 ] >70%) is a goal of all resuscitation interventions. If this
] >70%) is a goal of all resuscitation interventions. If this 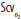 goal is not met by volume resuscitation and vasopressors, then red blood cell transfusion and inotrope infusion are the third and fourth interventions, respectively. When the goals of resuscitation are met, then reduction of vasopressor infusion, with further volume infusion if necessary, becomes a priority. CVP, central venous pressure; Hct, hematocrit.
goal is not met by volume resuscitation and vasopressors, then red blood cell transfusion and inotrope infusion are the third and fourth interventions, respectively. When the goals of resuscitation are met, then reduction of vasopressor infusion, with further volume infusion if necessary, becomes a priority. CVP, central venous pressure; Hct, hematocrit.
Early echocardiography is a useful adjunct to the above measurements to distinguish poor ventricular pumping function from hypovolemia; a good study can exclude or confirm tamponade, right heart failure, pulmonary hypertension possibly due to pulmonary embolism, or significant valve dysfunction, all of which influence therapy, and can replace more invasive pulmonary artery catheterization.
Volume Aggressive volume resuscitation up to the point of a heart that is too full is the first step in resuscitation of the circulation. The rate and composition of volume expanders must be adjusted in accord with the working diagnosis. The Early Goal-Directed Therapy algorithm for resuscitation of septic shock calls for 500 mL saline every 30 minutes, but this is much too slow in hypovolemic patients in whom 1 L every 10 minutes, or faster, is initially required. During volume resuscitation, infusions must be sufficient to test the clinical hypothesis that the patient is hypovolemic by effecting a short-term end point indicating benefit (increased blood pressure and pulse pressure and decreased heart rate) or complication (increased jugular venous pressure and pulmonary edema). Absence of either response indicates an inadequate challenge, so the volume administered in the next interval must be greater than the previous one. In obvious hemorrhagic shock, immediate hemostasis is essential11; blood must be obtained early, warmed and filtered; blood substitutes are administered in large amounts (crystalloid or colloid solutions) until blood pressure increases or the heart becomes too full. At the other extreme, a working diagnosis of cardiogenic shock without obvious fluid overload requires a smaller volume challenge (250 mL 0.9% NaCl in 20 minutes). In each case, and in all other types of shock, the next volume challenge depends on the response to the first; it should proceed soon after the first so that the physician does not miss the diagnostic clues evident only to the examining critical care team at the bedside during this urgent resuscitation (Table 33-3).
Urgent Resuscitation of the Patient With Shock—Managing Factors Aggravating the Hypoperfusion State
| Respiratory therapy |
| Protect the airway—consider early elective intubation |
| Prevent excess respiratory work—ventilate with small volumes |
| Avoid respiratory acidosis—keep PaCO2 low |
| Maintain oxygen delivery—FiO2, PEEP, hemoglobin |
| Infection in presumed septic shock (see Chap. 64) |
| Empirical rational antibiosis for all probable etiologies |
| Exclude allergies to antibiotics |
| Search, incise, and drain abscesses (consider laparotomy) |
| Arrhythmias aggravating shock (see Chap. 36) |
| Bradycardia |
| Correct hypoxemia—FiO2 of 1.0 |
| Atropine 0.6 mg, repeat × 2 for effect |
| Increase dopamine to 10 mg/kg per minute |
| Add isoproterenol (1-10 mg/min) |
| Consider transvenous pacer |
| Ventricular ectopy, tachycardia |
| Detect and correct K+, Ca2+, Mg2+ |
| Detect and treat myocardial ischemia |
| Amiodarone for sustained ventricular tachycardia |
| Supraventricular tachycardia |
| Consider defibrillation early |
| β-blocker, digoxin for rate control of atrial fibrillation |
| Sinus tachycardia 140/min |
| Detect and treat pain and anxiety |
| Midazolam fentanyl drip |
| Morphine |
| Detect and treat hypovolemia |
| Metabolic (lactic) acidosis |
| Characterize to confirm anion gap without osmolal gap |
| Rule out or treat ketoacidosis, aspirin intoxication |
| Hyperventilate to keep PaCO2 of 25 mm Hg |
| Calculate bicarbonate deficit and replace half if pH <7.0 |
| Correct ionized hypocalcemia |
| Consider early dialysis |
| Hypothermia |
| Maintain skin dry and covered with warmed blankets |
| Warm vascular volume expanders |
| Aggressive rewarming if temperature <35°C (95°F) |
Role of Red Blood Cell Transfusion During Initial Resuscitation Transfusion of red blood cells is a component of the initial volume resuscitation of shock when severe or ongoing blood loss contributes to shock. In addition, when anemia contributes to inadequate oxygen delivery so that mixed venous oxygen saturation, or its surrogate ScvO2 <70% despite an adequate CVP (8-12 mm Hg) and an adequate MAP (>65 mm Hg), then transfusion of red blood cells to hematocrit greater than 30% is a reasonable component of Early Goal-Directed Therapy and improves outcome.3 After initial resuscitation and stabilization, transfusion of red blood cells to maintain a hemoglobin above 90 g/L is no more beneficial than maintaining a hemoglobin level above 70 g/L and only incurs additional transfusion risk.12
Is There a Role for Delayed Resuscitation of Hypovolemia? During brisk ongoing hemorrhage, massive crystalloid or colloid resuscitation increases blood pressure and the rate of hemorrhage, so patient outcome may be worse.13 This does not mean that resuscitation is detrimental; rather, control of active bleeding is more important than volume replacement. Preventing blood loss conserves warm, oxygen-carrying, protein-containing, biocompatible intravascular volume and is therefore far superior to replacing ongoing losses with fluids deficient in one or more of these areas. Delayed or inadequate volume resuscitation, after blood loss is controlled, is likely a significant error that will have a detrimental effect on patient outcome.11
Vasopressors Whereas adequate cardiac output is more important than blood pressure (because adequate tissue oxygen delivery is the underlying issue), effective distribution of flow by the vascular system depends on an adequate pressure head. At pressures below an autoregulatory limit, normal flow distribution mechanisms are lost, so significant vital organ system hypoperfusion may persist in the face of elevated cardiac output due to maldistribution of blood flow. In this case, where inadequate pressure is the dominant problem, an assessment of organ system perfusion is made (urine output, mentation, and lactic acid concentration), and then a vasopressor agent such as norepinephrine is initiated to raise MAP.14 The increased afterload will decrease cardiac output, so this intervention as single therapy is appropriate only when cardiac output is high. If cardiac output and oxygen delivery are inadequate, then combination of vasopressor therapy with inotropic agents should be considered (see below).
Vasopressor therapy increases MAP and can increase cardiac output (venoconstriction increases venous return) and, therefore, often masks inadequate volume resuscitation and confounds the diagnosis of the etiology of shock. Thus, vasopressor use as part of Early Goal-Directed Therapy must be reassessed during ongoing volume resuscitation. Even when the numerical CVP and MAP goals have been attained, additional rapid volume challenge generally should be used to test for further clinical improvement (increased MAP, decreased heart rate, increased urine output, and increased ScvO2) and to determine whether this will allow titration of vasopressor use down or off.
Assessment of organ system perfusion (adequacy of organ function) is the most important component of vasopressor therapy; increase in blood pressure by itself is insufficient and can distract from careful reassessment of adequacy of oxygen delivery. If urine output increases, mentation improves, and lactate levels decrease, then vasopressor therapy has achieved its goals, and there is no need to increase MAP further even if the MAP that reverses these signs of hypoperfusion is 55 mm Hg. If the measures of organ system perfusion are not improved by vasopressor therapy, then arbitrarily driving MAP much above 70 mm Hg is rarely useful and usually detrimental because cardiac output will decrease further and excessive vasoconstrictor tone will impair blood flow distribution. If evidence of hypoperfusion persists, then inadequate volume resuscitation, cardiac output, hemoglobin, and oxygen saturation are more likely problems.
Which vasopressor is best? Recent randomized controlled trials (RCTs) show no significant survival benefit of any particular vasopressor in treating hypotension due to shock.15-18 However, these RCTs all show that increased β-adrenergic stimulation results in increased heart rate and increased incidence of arrhythmias (epinephrine>norepinephrine, dopamine>norepinephrine, norepinephrine>vasopressin). This adverse action of β-adrenergic stimulation may be important in some patients. Addition of low-dose vasopressin infusion to conventional norepinephrine infusion may improve survival in patients with less severe septic shock,18 particularly in patients with a mild degree of sepsis-induced renal dysfunction.19
Inotropes If evidence of inadequate perfusion persists (assessed by clinical indicators, by ScvO2, by direct measurement of cardiac output, etc) despite adequate circulating volume (Early Goal-Directed Therapy goal: CVP 8-12 mm Hg), vasopressors (Early Goal-Directed Therapy goal: >65 mm Hg), and hemotocrit, then inotropic agents are indicated2,3 (eg, dobutamine 2-20 μg/kg per minute). Inotropes are not effective when volume resuscitation is incomplete. In this case, the arterial vasodilating properties of inotropes such as dobutamine and milrinone result in a drop in arterial pressure that is not countered by an increase in cardiac output because venous return is still limited by the inadequate volume resuscitation. The corollary is, if initiation of inotropes results in a significant drop in blood pressure, then it follows that adequate volume resuscitation is not complete.
The objective of inotrope use is to increase cardiac output to achieve adequate oxygen delivery to all tissues. Organ function (mentation, urine output, etc) is the best measure. Of the many alternative clinical and laboratory indicators that should be measured, mixed venous O2 saturation (when a pulmonary artery catheter is placed) or ScvO2 is useful surrogate measures of adequacy of O2 delivery.20 Rapidly achieving a goal ScvO2 greater than 70% results in a substantial improvement in survival and limits the systemic inflammatory response so that the subsequent need for further volume, red blood cell transfusion, vasopressor use, and mechanical ventilation is reduced.3
Steroids Always controversial, steroids are currently not indicated for the treatment of shock and uniformly increase the incidence of superinfection.21
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree