Sex Hormones
Nancy E. J. Berman
Michael M. Behbehani
Millions of women suffer migraine headaches around the time of their menstrual cycles, a phenomenon known as menstrual migraine. Clinical studies completed over 25 years ago established strong links between falling blood estrogen levels and triggering of these attacks (126,127). This chapter addresses current knowledge of likely mechanisms linking hormones to migraine in both the peripheral and central nervous systems.
Migraine and other pain disorders, including fibromyalgia and temporomandibular disorders, are at least twice as prevalent in women as in men (34,73,76,128,156). In these conditions, severity of pain varies with the menstrual cycle, peaking around the time of menstruation when both estrogen and progesterone are lowest (54). Onset of migraine in girls usually occurs around time of menarche (128), and the frequency and severity of migraine attacks often increases during menopause, when hormone levels fluctuate. After menopause, when hormone levels are low, many migraineurs experience improvement (38). The constant high hormone levels of pregnancy are also associated with a decrease in migraine frequency (143). Thus, migraine improves during pregnancy, when estrogen levels are constantly high, and after menopause, when estrogen levels are constantly low. All of these observations indicate a strong link between ovarian steroids and migraine, and they suggest that rapid changes in estrogen and progesterone levels are a trigger for attacks. Ovarian steroids act via classical nuclear receptors such as ERα and ERβ, which regulate transcription by activating or repressing estrogen-responsive genes. Estrogen also activates elements of the MAP kinase pathway including extracellularsignaling related kinases (ERKs) (90). Other, more rapid effects of estrogen may be mediated via membrane estrogen receptors (70). Progesterone has two nuclear receptors, PR-A and PR-B, which are identical except for an additional 164 amino acids at the N-terminal end of PR-B (42). Progesterone also acts via membrane effects (144).
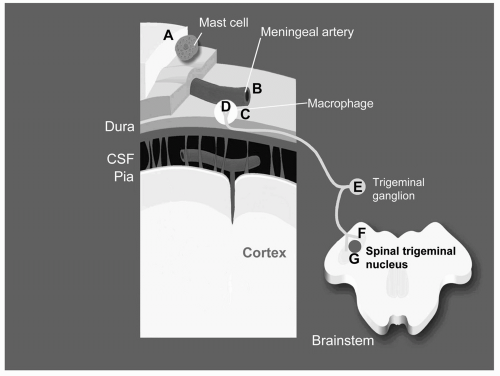
Hormones could affect pain processing at all levels, including peripheral and central mechanisms. Figure 17-1 diagrams regions where hormones could influence transmission of pain information from the peripheral into the central nervous system.
Peripheral Tissue
Meningeal Inflammation
Throbbing pain suggests an origin in arteries, and it has been known for many years that meningeal and large cerebral arteries are capable of transmitting painful sensations. Thus, studies of migraine pathogenesis have focused on meninges and meningeal vessels, and it is thought that migraine involves meningeal inflammation (95). There are at least four potential mechanisms whereby ovarian steroids could modulate meningeal inflammation. First, meningeal vessels are a potential site of estrogen effects, as they express estrogen receptor-α, which increases after estrogen treatment (129). Second, hormones may alter responses of meningeal mast cells. Estrogen receptors in dural mast cells modulate histamine release (109), and histamine excites nociceptive terminals. Third, estrogen may modulate expression of proinflammatory cytokines by macrophages present on the meningeal linings of the subarachnoid space (87) and in the dura, where they are aligned along blood vessels, especially near the superior sagittal sinus (85). Proinflammatory cytokines also excite nociceptive terminals (153). Estrogen modulates expression of proinflammatory cytokines in macrophages (68) and reduces leukocyte migration into injured vessels (153). Fourth, ovarian steroids may regulate sensory sensitization by modulating expression of nerve growth factor (NGF). Retrograde transport of receptor-bound NGF stimulates synthesis of CGRP, substance P, and PACAP (71,72,96). Blocking the high-affinity NGF receptor trkA prevents sensory sensitization following inflammation (14,64). Dural tissue expresses NGF (134), and estrogen treatment alters NGF expression in some tissues (16). Effects of NGF on meninges are unknown.
Trigeminal Neurons
Trigeminal Peripheral Processes
CGRP Innervation of Meninges
The dura is innervated by trigeminal axons containing substance P and CGRP. Migraine attacks involve selective release of CGRP from trigeminal axons (43), which causes plasma extravasation and meningeal edema (23,24,81). CGRP also functions as a potent vasodilator (101,138), stimulates mast cell histamine release (101), is highly chemotactic to macrophages (33), and increases cytokine release from macrophages (133). Estrogen increases CGRP innervation of mammary gland tissue, associated primarily with blood vessels (17). It is not known whether hormones have similar effects on CGRP innervation of meningeal vessels.
Trigeminal Neuronal Cell Bodies, Neuropeptides, and Hormone Receptors
The trigeminal ganglion contains several populations of neurons (see review by Lazarov [69]) that express neuropeptides such as substance P and CGRP, but also other peptides potentially relevant to migraine including PACAP, atrial natiuretic peptide, neurokinin A, endothelin-1, enkephalin, dynorphin cholecystokinin, bombesin, somatostatin, vasoactive intestinal peptide, and galanin (see review by Lazarov [69]). Trigeminal neurons express ERα, which is likely to regulate expression of many of these peptides.
Progesterone receptors are present in dorsal root ganglia (DRG) of female rats. Ovariectomy reduces expression of these receptors and increases behavioral
hypersensitivity to heat (93), suggesting a link between low progesterone levels and increased pain sensitivity. Progesterone may also regulate trigeminal function indirectly via effects on CGRP and the NGF-trkA system (41). Very little information is available about the potential role of progesterone or its receptors in trigeminal neurons.
hypersensitivity to heat (93), suggesting a link between low progesterone levels and increased pain sensitivity. Progesterone may also regulate trigeminal function indirectly via effects on CGRP and the NGF-trkA system (41). Very little information is available about the potential role of progesterone or its receptors in trigeminal neurons.
Hormones May Alter Responses to Peripheral Inflammation or Nerve Injury (Phenotypic Plasticity)
Numerous studies have shown that phenotypic changes in sensory neurons accompany changes in response to painful stimuli from temporary to severe and chronic. Cutaneous allodynia occurs in certain well-defined regions of the skin during migraine, suggesting hyperexcitability of pain pathways (22), and migraineurs have significantly lower thresholds for the blink reflex and an increased sensitivity to tactile and painful stimuli, even during the interictal period (115). These increased responses indicate sensitization of trigeminal neurons.
Phenotypic plasticity of nociceptive sensory neurons is regulated by the NGF-trkA system (88). Small DRG neurons that are positive for CGRP transport NGF from the periphery (1,152). During inflammation, NGF levels increase (110) resulting in inflammatory hyperalgesia (84,150, 151,152). Inflammation of orofacial tissue results in increased CGRP and substance P in the trigeminal ganglion (53). In addition, motifs that mimic a classical estrogen response element are found in the promoter and 5′-flanking regions of the genes for human trkA (125,135).
Sensory sensitization is associated with altered expression of neuropeptides (NP) such as NPY and galanin in trigeminal neurons in males (35,40). In females, NPY and galanin are regulated by ovarian steroids. NPY is a vasoconstrictive peptide usually associated with the parasympathetic nervous system. NPY-immunoreactive nerve fibers and receptors are expressed around cerebral vessels (12), and NPY inhibits dural plasma protein extravasation after trigeminal stimulation (155). Increased levels of NPY are present in cerebral spinal fluid after a migraine attack (139). Both Y1 and Y2 NPY receptors are located in trigeminal ganglia (131). NPY mRNA levels vary with the phase of the estrous cycle in female mice, with highest levels present at early estrus (104). Galanin increases markedly after nerve injury (49), when it regulates nociceptive signaling (50,62,63,75). In male mice, injury increases the galanin content of dorsal root ganglion cells 120-fold and the number of galanin positive neurons increases from 5% to more than 50% (2). Galanin injections into inflamed knee joints in rats cause a significant reduction in responses to noxious movements, suggesting that galanin is a critical component of the tonic inhibitory system for inflammatory pain (47). In the pituitary, estrogen upregulates galanin mRNA expression up to 4000-fold and immunoreactivity up to 50-fold (59). In mice, galanin expression in trigeminal neurons is highest at proestrus, the stage of the estrous cycle when estrogen levels are highest (104). Galanin has several G-protein-coupled receptors including GalR1, GalR2, and GalR3 (20). GalR1, which has antinociceptive functions (52,61,74), is expressed in trigeminal ganglia and in peripheral targets of trigeminal neurons (130). GalR1 expression in the hypothalamus is modulated by ovarian steroids and is highest at proestrus (37). It is not known if ovarian steroids regulate GalR1 expression in the trigeminal system.
Intracellular Signaling Pathway
Inflammatory mediators produce hyperalgesia via activation of intracellular signaling pathways including members of the MAP kinase pathway ERKs (55). Activation of nociceptive fibers induces phosphoERK (pERK) in sensory neurons in an intensity-dependent manner, and ERK antagonists inhibit capsaicin-induced hyperalgesia (31). Ovarian steroids can modulate the activity of the ERK pathway (67).
Potential Effects of Ovarian Steroids on Trigeminal Central Processes and Postsynaptic Cells
Inflammation increases in the number of substance P and CGRP immunoreactive axons in the dorsal horn, whereas nerve injury increases galanin and NPY (51). The extent of neuroplasticity of trigeminal central terminals and potential effects of ovarian steroids on these responses have received little attention. Ovarian steroids may also regulate activity of postsynaptic cells directly, as neurons in lamina II of trigeminal nucleus caudalis (TNC) express ERα, and the number of these cells increases after ovariectomy (102). ERα is also present in nucleus caudalis (123). Changes in excitability of brainstem trigeminal neurons may underlie the increases in receptive field sizes in the spinal trigeminal nucleus observed when estrogen levels are high (9,10).
INTERACTIONS OF SEX HORMONES WITH BRAIN PAIN PROCESSING NETWORKS
Overall Network
The information regarding the peripheral action of sex hormones has been obtained from experimental animals. This is because there are no noninvasive techniques that can provide this type of information using human subjects. More data on the role of sex hormones in the
central processing of trigeminal pain processing in human are known because of the advances in imaging techniques. The currently available information from human studies correlates well with the animal studies of the role of sex hormones.
central processing of trigeminal pain processing in human are known because of the advances in imaging techniques. The currently available information from human studies correlates well with the animal studies of the role of sex hormones.
Information related to activation of sensory terminals innervating the dura and meninges are transmitted to neurons within the nucleus caudalis of the trigeminal complex. Functionally, this region, and its caudal extent into the cervical spinal cord, integrate nociceptive information arriving from the periphery and the descending systems and transmits this information to the medullary, pontine thalamic, and hypothalamic regions (11,26,118). Anatomic studies using injection of retrograde tracers into the TNC have identified four major sites as targets of TNC projections (26,32,46,78, 79, 80,94,99,107,108,119,148). These sites are the periaqueductal gray (PAG), the parabrachial nucleus (PBN), medial preoptic nucleus of the hypothalamus, and the ventrobasal nucleus of the thalamus. Further, injections of anterograde tracers into each of these sites indicate site-specific afferents from the TNC neurons that in turn receive afferents from the meninges and dura. The sites that receive afferents from TNC contain a dense population of sex hormone receptors and terminals. Therefore, changes in the level of these hormones can modulate signal processing within the TNC and propagation of these signals to the forebrain. The functions related to sex hormones in each of these sites are discussed next.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree