FIGURE 4.1 Schematic diagram of the intracranial pressure (ICP)–volume relationship (elastance). As intracranial volume increases (A to B) compensation occurs resulting in minimal increase in ICP, but as elastance decreases there is a marked increase in ICP (C to D) for even small increases in intracranial volume. Further increases in ICP will result in decrease cerebral perfusion pressure (CPP), global ischemia, and herniation. CBF, cerebral blood flow.
2. Blood–brain barrier. The blood–brain barrier is impermeable to electrolytes; however, water will shift between blood and brain compartments according to osmotic gradients, particularly serum sodium concentrations (see Chapter 3 for details). Intracranial lesions and injury may disrupt the blood–brain barrier allowing water, electrolytes, and osmotic molecules to crossover producing brain edema.
3. Cerebral blood flow (CBF)
a. CBF is coupled with cerebral metabolic requirement of oxygen (CMRO2) and is controlled by cerebral autoregulation, cerebral perfusion pressure (CPP), cerebrovascular resistance (CVR), and cerebrovascular reactivity to CO2 (see Chapter 1 for details).
b. Cerebral autoregulation is the ability of cerebral vessels to provide a constant CBF (=50 mL/100 g/min of brain weight) over a wide range of mean arterial pressure (MAP). This range is variable among individuals with a lower limit of MAP of approximately 70 mm Hg (Fig. 4.2). In untreated or poorly controlled chronic hypertension, the autoregulation range will shift toward higher values.
c. CO2 is a cerebral vasodilator/constrictor. A linear relationship exists between PaCO2 (25 to 80 mm Hg) and CBF. CBF changes from 2% to 4% for each mm Hg change in PaCO2. Excessive hyperventilation may lead to ischemia especially in areas of compromised CBF.
d. CPP is the difference between MAP and ICP.
e. Autoregulation and CO2 reactivity can be disturbed by various pathologic states (injured brain, tumor) and also anesthetic agents [1,2].
CLINICAL PEARL
The concept of the range of autoregulation is an evolving concept in which the upper and lower limits are more likely to be a “rounded shoulders” than a “sharp elbows.” The lower limits may be as high as a 25% decrease from baseline and cerebral hypoperfusion may occur when the MAP is 40% to 50% of the resting value.
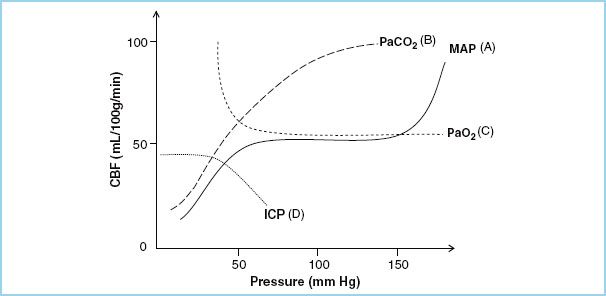
FIGURE 4.2 This is an illustration of the relationships of cerebral blood flow (CBF) to changes in (A) mean arterial blood pressure (MAP), known as the cerebral autoregulation curve, (B) carbon dioxide (PaCO2), (C) oxygen (PaO2) tensions, and (D) intracranial pressure (ICP).
4. Anesthetic effects
a. Volatile anesthetic agents
(1) Volatile anesthetic agents cause cerebral vasodilation (decreased CVR) that increases CBF resulting in increases in CBV and ICP. These agents also have a dose-related decrease in CMRO2 (see Chapter 2 for details).
(2) Volatile agents should be kept at <1 MAC to maintain CO2 reactivity and autoregulation in normal brain tissue.
(3) Nitrous oxide may increase CMRO2 with an increase in CBF and, thus may increase ICP.
b. Intravenous anesthetic agents
(1) Most intravenous agents (thiopentone, propofol) are potent cerebral vasoconstrictors through their ability to reduce CMRO2. Due to tight coupling of CBF to CMRO2 the decrease in CMRO2 results in an increase in CVR with a reduction in CBF, CBV, and thus ICP.
(2) Ketamine is the exception as it increases CBF.
(3) Opioids (fentanyl, remifentanil, sufentanil) cause a modest reduction in CMRO2 but do not affect autoregulation or CO2 reactivity. However, respiratory depression that results in marked hypercarbia will increase CBF and CBV. In addition, hypotension resulting from large bolus administration of narcotics may cause reflex cerebrovasodilation with an increase in CBV and potentially ICP.
(4) Neuromuscular blocking agents generally have minimal effect on CBF. However, succinylcholine may cause transient limited increases in ICP under conditions of intracranial hypertension. These ICP changes can be blocked by preadministration of a defasciculating dose of nondepolarizing relaxants, hyperventilation, or an intravenous anesthetic agent.
C. Pathology of lesions
2
1. Tumors
a. In adults most primary and metastatic tumors are in the supratentorial compartment (80%); in children they are more frequently infratentorial.
b. Primary tumors (60% of tumors) range from benign well differentiated to anaplastic. Surgical management usually consists of peritumor edema control (24 to 48 hours of course of corticosteroids) and tumor debulking.
c. Glioblastomas are the most common and have a rapid progression leading to symptoms of increased ICP and focal deficits with poor prognosis.
d. Oligodendrogliomas are less common, slow growing, and often calcified presenting with seizures or localizing signs.
e. Astrocytomas may present with a long history of seizures or late-onset focal deficits.
f. Meningiomas arise from the meninges and are classically benign, causing compression rather than invasion. They have a high rate of local recurrence and may be very large and highly vascular. Preoperative embolization is used at times to decrease intraoperative bleeding.
g. Metastatic tumors are common and develop from lung, colon, breast, or kidney cancer. They occur in about 25% of patients with cancer.
2. Brain abscess
a. An abscess may cause similar effects on the brain as tumors (increased ICP). The patient may be febrile and have a gradual onset of meningitis.
b. They often result from local spread of a source of infection (frontal sinus or middle ear) or blood-borne infection.
c. Close communication with the surgical team is required regarding the timing of the administration of antibiotics.
3. Intracranial hematoma
a. Subdural hematomas
(1) May present as either an acute, subacute, or chronic hematoma. Symptoms will depend on how quickly they develop. Patient may also have an acute on chronic bleed.
(2) Chronic subdural hematomas usually affect the elderly. Symptoms (confusion, drowsiness, and fluctuating level of consciousness) may present weeks after the initial injury.
(3) If acute in nature, there is less time for the brain to accommodate.
(4) These lesions are treated with burr hole aspiration or minicraniotomy.
b. Epidural hematoma
(1) An acute epidural hematoma occurs when blood accumulates between the skull and dura. This usually occurs as the result of a traumatic insult that causes a skull fracture and rupture of the underlying blood vessels (usually an artery).
(2) Neurologic decompensation occurs rapidly (often within hours) and the patient may be both neurologically and hemodynamically unstable.
(3) These patients may require rapid treatment of increased ICP, including airway and ventilatory management. Since the brain parenchyma is usually not injured, the prognosis is excellent if the hematoma is rapidly decompressed.
c. Intracerebral hematoma
(1) An intracerebral hematoma (hemorrhage) results from bleeding into brain tissue. Etiology is usually related to rupture of normal vessels due to high blood pressure or abnormal blood vessels such as an arteriovenous malformation, an aneurysm, or bleeding into a tumor.
(2) Symptoms usually are the sudden onset of a stroke.
(3) Treatment includes management of blood pressure, ICP, and occasional surgical/intra-arterial interventions depending on the primary pathology.
D. Surgical procedures. Patients may undergo a biopsy, curative resection, or debulking of a supratentorial lesion.
1. Stereotactic surgery
a. Is a minimally invasive procedure that uses a three-dimensional coordinate system to locate small lesions inside the brain and is often used for obtaining a biopsy.
b. These procedures may be performed with either general anesthesia or conscious sedation.
(1) Continuous vigilance is needed to diagnose and treat complications quickly.
(2) A sudden change in neurologic status or decreased level of consciousness may result from intracranial bleeding or neurologic injury.
(3) Respiratory changes may occur from oversedation and/or from an intracranial event.
c. May be “frame-based” which involves the placement of a light-weight headframe with a base that is attached to the cranium with skull pins. Computerized tomography (CT) or magnetic resonance imaging (MRI) then identifies the targeted lesion in relation to reference points on the external frame. Surgical apparatus is attached to the headframe.
(1) Placement may occur in the operating room or in the imaging suite.
(2) This can be performed with local anesthesia with or without sedation.
(3) If general anesthesia is used, then most often the frame is placed after anesthesia induction.
(4) The headframe may obscure the patient’s mouth and/or nose and may limit extension of the neck, making airway manipulation difficult.
(5) During the procedure, the stereotactic headframe is attached to the operating room table. This results in lack of ability of the patient to move their head and may result in a compromise of the patient’s airway.
d. A “frameless” stereotactic system is now frequently used with placement of external fiducial scalp markers, facial surface features, or a set of marks in the reticule of an optical instrument for points of reference. The use of a head holder frame attached to the operating room table may still be required. If the patient is awake for placement, local anesthesia will be infiltrated at the pin sites.
CLINICAL PEARL
Since stereotactic surgery often relies on precise coordinates from external references, high resolution neuroimaging, and/or advanced computer systems, preventing changes in preoperative brain parenchymal relationships due to changes in PaCO2, or the administration of osmotic diuretics is paramount to the success of these minimally invasive techniques.
2. Craniotomy
a. For the excision of a lesion a craniotomy is performed with a lateral pterional, temporal, or frontal surgical approach.
b. Minimally invasive surgery and endoscopic approaches are also used.
c. A bifrontal craniotomy increases the risk of venous air embolism (VAE) due to close proximity to the sagittal sinus (see Chapter 22).
d. Intraoperative MRI is being used to improve patient outcome with better resection of tumors.
II. Anesthetic management for craniotomy. The overall goals for the anesthetic management begin with full understanding of the patient’s neurologic condition and planned procedure (Table 4.1) [3].
Table 4.1 Goals of anesthetic management

3
A. Preoperative assessment
1. Purpose
a. Review of patient’s medical history and findings.
b. Optimize patient’s overall medical status.
c. Prepare a plan for the anesthetic management.
2. Presenting symptoms
a. Many tumors grow slowly allowing for adaptive mechanisms before becoming symptomatic.
b. Symptoms may be generalized with signs and symptoms of increased ICP (headache, nausea, vomiting, and changes in mental status).
c. Or symptoms may be localized with seizures or focal deficits.
d. Tumors may be surrounded by edema that responds to corticosteroid treatment. Frequently, at the time of surgery a patient’s symptoms may have completely disappeared.
3. Neurologic assessment
a. History of disease including presenting symptoms, and the type, location, and size of lesion.
b. Neurologic status: Level of consciousness, Glasgow Coma Scale score (GCS), any signs and symptoms of increased ICP.
c. History of seizures.
d. Presence of focal deficits (such as hemiparesis).
4. General medical condition
a. Review and optimize cardiac and respiratory systems.
b. Assessment of other comorbidities such as diabetes, renal impairment, hematology, and allergies.
c. Malignant tumors may cause coagulation disorders with an increased risk of thromboembolism. Low molecular heparin may be used in these patients postoperatively.
d. Airway assessment.
5. Medications
a. All routine medications (cardiovascular, respiratory, and gastrointestinal) and especially anticonvulsants, and steroids (dexamethasone) should be continued preoperatively.
b. Appropriate treatment of diabetic patients.
6. Laboratory
a. Routine blood work including hemoglobin, blood glucose level, and creatinine.
b. ECG is useful as neurologic diseases can cause a variety of nonspecific ECG changes.
c. Crossmatched blood (2 to 4 units) should be available if excessive blood loss is anticipated.
7. Imaging
a. CT and MRI
(1) For assessment of type, location, and size of lesion.
(2) May indicate vascularity of the tumor.
(3) Proximity of tumor to venous sinuses (risk of VAE).
(4) Signs of increased ICP such as midline shift, edema, ventricular distortion, or hydrocephalus.
(5) To determine the ease of surgical access and patient positioning.
b. Metabolic imaging. Positron emission tomography, magnetic resonance spectroscopy, and single photon emission tomography will give more precise information on the size and location of the tumor.
c. Chest x-ray. Assessment of respiratory diseases and of any primary cancer.
B. Anesthetic management
4
1. Premedication
a. Preoperative sedation should be avoided in patients with increased ICP, low GCS, and with large mass lesions.
b. Sedation may result in depressed respiratory efforts leading to elevated PaCO2 and increase in CBF, CBV, and ICP.
c. When premedication is used it must be administered with continuous observation and monitoring of the patient.
d. Particularly anxious patients may be premedicated with a short-acting benzodiazepine with the above precautions.
2. Monitoring
a. All standard monitors should be used. Additional monitoring as indicated by requirements of patient and procedure.
b. Vascular access for large craniotomy requires two large bore intravenous lines (18G or larger).
c. Invasive cardiovascular monitoring
(1) Invasive intra-arterial blood pressure for most patients is indicated. In unstable patients, this should be placed preinduction using local anesthesia. Arterial reference should be at the level of the middle ear/circle of Willis.
(2) Central venous pressure (CVP) monitoring may be useful in procedures with a large estimated blood loss (tumors encroaching venous sinuses, large vascular tumors such as meningioma), possibility of VAE and in procedures or patients with marked fluid shifts, and patients with difficult venous access.
(3) Pulmonary artery pressure monitoring is rarely required.
d. Urinary catheter is needed for most procedures, especially when mannitol or diuretics are used or excessive urine output is expected (e.g., diabetes insipidus).
e. Core temperature monitoring with a nasal-pharyngeal or esophageal probe is indicated.
f. Warming blankets and blood warmers are useful as indicated to ensure normothermia.
g. Routine ICP monitoring is rarely used. When the cranium is open, observation of conditions of the surgical field provides useful information (presence or lack of dural bulging). Lumbar CSF drains are rarely used for tumor surgery, but if present may be used to monitor lumbar CSF pressure.
h. Neurophysiologic monitoring with somatosensory and/or motor evoked potentials, electroencephalography, and electromyography may be used (see Chapter 26).
i. Other monitors used in specific patients include monitoring neuromuscular blockade, biologic (coagulation, hematology, blood gases, glucose), jugular venous bulb oxygen saturation, transcranial Doppler, transcranial oximetry, precordial Doppler (for VAE), depth of anesthesia, and microvascular ultrasonic blood flow probe.
3. Induction of anesthesia
a. Induction of anesthesia must be smooth and controlled with hemodynamic stability. Adequate depth of anesthesia, particularly at intubation, is important to prevent hypertension. Preventing hypoxemia, hypercarbia, and increases in venous pressure can minimize increases in ICP.
b. Anesthesia induction can be achieved by using titrated doses of induction agents (midazolam, propofol, thiopentone) and an opioid (fentanyl 2 to 3 μg/kg, sufentanil 0.2 to 0.3 μg/kg, remifentanil 0.5 to 1.0 μg/kg) followed with short-acting nondepolarizing muscle relaxants (rocuronium, vecuronium, cisatracurium). Succinylcholine may be used depending on the risk benefit of rapid airway control versus the small risk of transient increases in ICP.
(1) Etomidate may be useful in hemodynamically unstable patients.
(2) Addition of lidocaine 1 to 1.5 mg/kg IV will help to blunt the laryngeal reflex to intubation and reduce ICP by cerebral vasoconstriction.
(3) Prior to laryngoscopy and intubation, the depth of anesthesia should be deepened with an additional bolus of anesthetic agents (propofol or opioid) or β-blocker (esmolol 0.5 to 1.0 mg/kg) or antihypertensive (labetalol 0.25 to 0.5 mg/kg) to suppress sympathetic response.
c. Other helpful measures include voluntary hyperventilation by the patient while awake and mild hyperventilation during induction.
4. Positioning
a. Final positioning of the patient must be reviewed by both the surgeon and anesthesiologist.
b. Supine position with or without a shoulder roll to turn the head away from surgical site is used for most procedures.
c. For some procedures a full lateral position is used.
d. The endotracheal tube should be securely placed on the appropriate side. A soft bite block may be useful.
e. Prior to head fixation with skull pins the depth of anesthesia must be deepened to prevent hypertension. This can be with additional propofol (0.5 mg/kg), fentanyl (2 to 3 μg/kg), or remifentanil (0.5 to 1.0 μg/kg), with or without injection of local anesthesia (lidocaine) at site of pin insertion.
f. Extreme head rotation and flexion may obstruct internal jugular veins and affect venous drainage causing an increase in ICP.
g. Slight head up will optimize venous drainage.
h. Patients’ eyes must be securely taped and protected from desiccation or irritation from skin preparation solutions.
i. Care is needed for all pressure points and the prevention of nerve compression.
j. Thromboembolism prophylaxis should be considered. Elastic stockings or sequential pneumatic calf compression devices may be used.
5. Maintenance of anesthesia
a. Pharmacologic management
(1) Both total intravenous anesthesia (TIVA) and inhalation techniques have been used. The choice is mostly at the discretion of the anesthesiologist. No major differences in outcome of the patient have been shown between these techniques [4–6].
(2) Inhalation-based anesthesia
(a) Inhalation anesthesia used in conjunction with opioids is easily controllable and allows for early awakening.
(b) Inhalation agents (isoflurane, desflurane, and sevoflurane) cause cerebrovasodilation and thus may increase CBF and ICP resulting in brain swelling.
(c) However at a < 1 MAC CO2 reactivity remains intact and control of vasodilator effects of volatile agents can be minimized with hypocarbia in normal brain tissue.
(d) Nitrous oxide (N2O) increase CMRO2 which may increase CBF and ICP. Hypocapnia and intravenous agents minimize the cerebral vasodilation. N2O use, though still controversial, is declining [7]. It should not be used in patients with a recent craniotomy as N2O diffuses into air-containing space leading to pneumocephalus.
(3) TIVA
(a) The most common TIVA agents used are an opioid and propofol. Propofol reduces CMRO2, CBF, CBV, and ICP, and preserves CO2 reactivity.
(b) TIVA may result in prolonged and unpredictable awakening.
(c) TIVA is indicated in the patient who has high ICP and significant brain swelling.
(4) Opioids
(a) Opioids (fentanyl, sufentanil, remifentanil) add to hemodynamic stability and reduce the requirement for other anesthetic agents.
(b) Remifentanil allows more rapid emergence as it is easily titrable, and its metabolism gives it a short context-sensitive half-time.
(5) Neuromuscular blockers
(a) Muscle relaxation may be used with intermittent bolus or infusion of short- or medium-duration agents: rocuronium, vecuronium, cisatracurium.
(b) Muscle relaxation should be avoided when neurologic monitoring includes motor evoked potentials or electromyography.
(c) Higher doses of remifentanil lessen the risk of movement in the absence of muscle relaxants [8].
(6) Dexmedetomidine (0.2 to 1.0 μg/kg/h)
(a) An α-2 adrenoreceptor agonist that has sedative, sympatholytic, and analgesic properties [9,10].
(b) Provides hemodynamic stability during general anesthesia by decreasing hemodynamic responses to noxious stimuli and during surgery and emergence.
(7) Adjunctive medications
(a) Administration of antibiotics, steroids (dexamethasone), and antiseizure prophylaxis (levetiracetam, fosphenytoin, phenytoin) is given as indicated and repeated if necessary during prolonged procedures.
(b) A loading dose of phenytoin (15 mg/kg) must be administered slowly to prevent hemodynamic instability.
(c) Furosemide (10 to 20 mg), mannitol (0.5 to 1.0 g/kg), or hypertonic saline solutions (3 mL/kg 3% NaCl) may be used to reduce intracranial volume and ICP. With NaCl plasma sodium of up to 160 mmol/L is targeted to control ICP.
(d) Antiemetics for postoperative nausea and vomiting (PONV) prophylaxis should be given. Most commonly, one of the 5-HT3 receptor antagonists are used (ondansetron, granisetron, dolasetron, tropisetron, or palosetron) [11,12].
b. Ventilation
(1) Mild to moderate hyperventilation (PaCO2 25 to 35 mm Hg) results in cerebral vasoconstriction and a reduction in CBF, CBV, and ICP. This is an efficient and rapid means of providing a slack brain and lessening the need for excessive harmful retraction of the brain [13,14].
(2) A PaCO2 measurement is useful to correlate to end-tidal CO2.
(3) Changing back to normoventilation should be done with caution as an increase in ICP may occur.
(4) If required, the use of positive end expiratory pressure (PEEP) must be with caution to prevent decrease in cardiac output and increases in ICP.
c. Optimization of surgical conditions. Anesthetic maneuvers are important in the production of a “slack” brain or to reduce brain bulk to allow for ease of surgical access to the lesion without compromising cerebral perfusion and to prevent ischemic injury (Table 4.2).
Table 4.2 Optimization for reduction of brain bulk

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







