INTRODUCTION AND EPIDEMIOLOGY
Cardiac arrest in pregnancy is rare, and resuscitation of a pregnant woman is typically an unexpected and chaotic event, which ideally involves multiple consultants from different specialties with different levels and types of skills. Emergency care and lifesaving procedures for resuscitation and cardiac arrest should not be delayed if specialists are not available. Contact the closest center providing neonatal and maternal services as soon as possible to facilitate rapid transport and continued care of the newly delivered infant and the mother.
The World Health Organization defines maternal deaths as deaths while pregnant or within 42 days of the end of pregnancy, related to or aggravated by pregnancy or pregnancy management, regardless of the duration or site of the pregnancy and irrespective of the cause of death.1 Factors associated with pregnancy-related deaths in the United States include advanced maternal age, African American race, increasing live birth order, and lack of prenatal care.2
Management of emergencies during labor and delivery and diagnosis and management of pulmonary embolism and eclampsia are discussed in the chapters 101, “Emergency Delivery” and 100, “Maternal Emergencies after 20 Weeks of Pregnancy and in the Postpartum Period.”
PHYSIOLOGY OF PREGNANCY
Beginning early in pregnancy, virtually all major organ systems undergo changes (Table 25-1) that affect patient management.
| System | Parameters | Comment |
|---|---|---|
| Cardiovascular | Cardiac output | Increases 30%–50% |
| Peripheral resistance | Decreases 20% | |
| Blood pressure | Decreases 10–15 mm Hg systolic in first half of pregnancy; then back to baseline | |
| Blood volume | 100 cc/kg or 6–7 L | |
| Central venous pressure | May be increased up to 10 mm Hg | |
| Central venous oxygen saturation | Increases as high as 80%3 | |
| Plasma volume | Increases 30%–50% | |
| Hematologic | Fibrinogen, factors V, VII, VIII, X, von Willebrand factor | Increase, with heightened risk for venous thromboembolism in second half of pregnancy |
| Respiratory and pulmonary | Upper airway edema, hyperemia, and friability | Estrogen and volume effects; can result in difficult airway |
| Diaphragm elevation | Higher thoracostomy tube insertion site during pregnancy | |
| Hemoglobin F has greater affinity for oxygen than maternal hemoglobin | Fetal oxygen maintained at expense of maternal oxygenation; maintain maternal oxygen saturation <95% | |
| Respiratory rate | No change | |
| Tidal volume, minute ventilation | Increase | |
| Renal and urinary | Progesterone dilates renal collecting system; ureteral peristalsis decreases | Renal US may show mild hydronephrosis; increased risk for ascending infection |
| GI | Alkaline phosphatase rises from placental production; bile is more lithogenic | Increased risk of cholecystitis/cholelithiasis |
| Decreased lower esophageal tone; decreased gastric emptying | Increased likelihood of aspiration of gastric contents | |
| Uteroplacental unit | 25% of blood flow directed to uteroplacental unit; no autoregulation of blood flow; enlarging uterus can compress vena cava and vessels below the diaphragm; supine hypotension syndrome can occur after 30 min of supine position | Place patient in left lateral tilt position during third trimester; replace volume adequately to account for increased blood and plasma volume in pregnancy; avoid femoral and lower extremity site for blood and volume delivery in second half of pregnancy |
Uterine blood flow is not autoregulated but is directly proportional to the maternal mean arterial pressure and inversely proportional to the resistance of the uterine vasculature. Conditions that decrease uterine blood flow during pregnancy include maternal hypovolemia, hypotension, uterine vasoconstriction, tetanic uterine contractions and aortocaval compression.
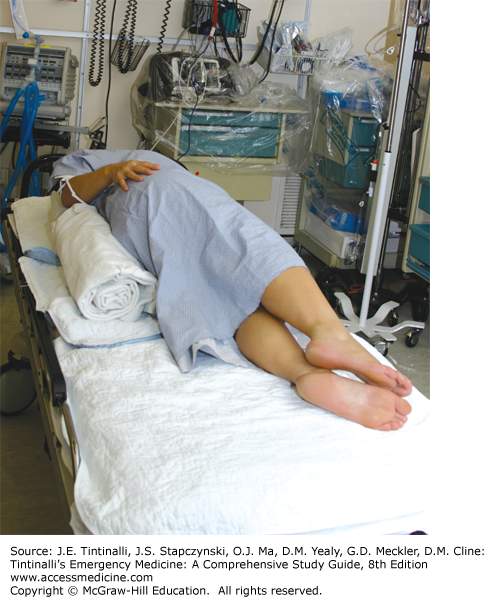
As the uterus enlarges throughout pregnancy, compression of the pelvic and abdominal vasculature can occur, especially when the patient is supine. Compression of the inferior vena cava can decrease maternal venous return and reduce cardiac output from 10% to 30% (Figure 25-1).4 This can contribute to supine hypotension syndrome, which is a constellation of findings including hypotension, tachycardia, dizziness, pallor, and nausea. It is reported to occur after 30 minutes in the supine position.4 Therefore, place any patient in the third trimester of pregnancy in the full left lateral tilt position when in hemodynamic distress or exhibiting hypotension.5 Left lateral tilt can be achieved by placing a roll under the patient’s right hip, insertion of a Cardiff wedge, or full left lateral tilt of the patient while on a backboard (Figure 25-2). The Cardiff wedge provides a tilt of 27 degrees from the horizontal. Foam or hard wedges are better for maintaining the left lateral tilt position than are pillows or manual tilting.6 Because compression of the abdominal vasculature can compromise intravascular delivery of medications through sites below the diaphragm, avoid femoral or saphenous venous sites for IV access during the resuscitation of a pregnant woman at >20 weeks of gestation. The negative effects of great vessel compression on uteroplacental blood flow increase in the presence of maternal hypotension and uterine contractions.
FIGURE 25-1.
Changes in maternal heart rate, stroke volume, and cardiac output during pregnancy (preg.), with the gravida in the supine and lateral positions. PP = postpartum. [Reproduced with permission from Barclay ML: Critical physiologic alterations in pregnancy, in Pearlman MD, Tintinalli JE (eds): Emergency Care of the Woman. New York, The McGraw-Hill Companies, Inc., 1998, Chapter 2, Figure 2-3, p. 14. Copyright © 1998 by The McGraw-Hill Companies, Inc. All rights reserved.]
Respiratory changes occur early to help optimize fetal oxygenation. Progesterone drives an increase in resting minute ventilation and tidal volume. The respiratory rate, however, remains relatively unchanged, so do not dismiss tachypnea as a normal part of pregnancy. As pregnancy progresses, the diaphragm elevates approximately 4 cm with an increase of the transverse diameter of the thoracic cage by 2 cm.7 Changes in pulmonary physiology and increased metabolic oxygen consumption can result in the rapid development of hypoxia during respiratory illnesses or as a result of respiratory arrest.
Because the fetal oxyhemoglobin dissociation curve is shifted to the left relative to the maternal oxyhemoglobin dissociation curve, the bond of fetal hemoglobin to oxygen is stronger than maternal hemoglobin, which results in preservation and optimization of fetal oxygen delivery at any given PO2. Strive to maintain maternal pulse oximetry readings >95% to maintain a PaO2 of >70 mm Hg to optimize maternal oxygenation and oxygen delivery to the placenta.7
The fetus exists in a physiologically acidemic state relative to the mother, which allows preferential oxygen transfer at the fetal tissue level.8 Acidemia favors a rightward shift of the oxyhemoglobin dissociation curve, resulting in a greater amount of oxygen supplied to fetal tissues. Fetal cardiac output protects against brief periods of hypoxia, with increases in umbilical blood flow, placental gas exchange, and preferential redistribution to vital tissues.
RESUSCITATION IN PREGNANCY
The most common causes of pregnancy-related deaths in the United States are cardiovascular disease, cardiomyopathy, hemorrhage, infection/sepsis, hypertensive disorders of pregnancy, and thrombotic pulmonary embolism.2,9,10 Major trauma is the greatest risk for nonobstetric cause of death. Motor vehicle collisions account for almost half of trauma, followed by falls and assaults.11
The best maternal care will provide the best fetal care, so follow general principles of resuscitation when treating pregnant women. To accommodate the increase in blood and plasma volume that develops during pregnancy, make sure that the volume of resuscitative fluids increases by 50% above that required by the nonpregnant patient. Place two large-bore IVs, and provide rapid infusions of isotonic saline. Volume must be adequately replaced before considering vasopressors, especially in pregnancy, because the uterine arteries are maximally dilated and blood flow is pressure dependent.
Select a vasopressor by the desired effects in the mother and the least harmful effects on the fetus (see Table 25-4). Because of variable effects of vasopressors on fetomaternal circulation, obtain critical care and obstetrical consultation if possible, before selecting vasopressors.
Studies of vasopressors in pregnancy focus on the treatment of hypotension with pseudoephedrine or ephedrine during spinal anesthesia for cesarean section or involve animal models, and data are difficult to extrapolate to other human hypotensive situations. Phenylephrine (pregnancy risk factor C) is an α1 selective agent without any β activity, so it raises blood pressure but decreases heart rate. It crosses the placenta and is excreted in breast milk but has a favorable fetal acid-base profile. Ephedrine (pregnancy risk factor C) is a mixed α and β stimulator. It crosses the placenta and can induce fetal acidosis.12,13 The vasopressors norepinephrine, dopamine, and vasopressin are all pregnancy risk factor C with limited data available on use in pregnancy.
Sepsis is a systemic inflammatory response to infection, leading to acute organ dysfunction. Currently there are no sepsis guidelines specifically for pregnant women, because this population has been excluded from early landmark sepsis studies. Pregnant women, when compared with nongravid women, are more likely to develop complications from serious infections. Maintain a high index of suspicion for sepsis in pregnant women, because signs and symptoms of sepsis may not be as apparent when compared to the nonpregnant population.14 The clinical features of sepsis in pregnancy include fever or rigors, diarrhea or vomiting, rash, abdominal or pelvic pain, vaginal discharge, productive cough, or urinary symptoms.15
Septic shock in pregnancy is rare, occurring in a small number (0.002% to 0.01%) of deliveries and in only 0.3% to 0.6% of pregnant women (Table 25-2). However, sepsis as a cause of mortality can vary between 2.7% in developed countries to 11.6% in developing countries.16 Common causes of sepsis are pyelonephritis, pneumonia, chorioamnionitis, and septic abortion.17 Malaria, human immunodeficiency virus, and community-acquired pneumonia are causes of sepsis in developing countries.18
Pyelonephritis is the most common cause of septic shock in pregnancy.17 Progesterone produces dilatation of the ureters, and mechanical compression of the urinary system by the enlarging uterus results in relative obstruction of the urinary tract. The most common causative agent is Escherichia coli, with Klebsiella, Proteus, and Enterobacter responsible for most other cases. Consider renal US to assess for renal/ureteral stones and to assess for renal complications.19 Hospitalize pregnant women with pyelonephritis, because bacteremia is likely and the physiologic changes of pregnancy can rapidly cause hypoxia.20,21 Begin empiric antibiotics. Treatment needs to consider local resistance patterns. In general, for mild to moderate disease, standard regimens are amoxicillin/clavulanate, ampicillin plus gentamicin, ceftriaxone, or cefepime. For severe disease with immunocompromise, consider ticarcillin/clavulanate or piperacillin/tazobactam.
Pneumonia in pregnancy can be particularly severe because a rapid decline in oxygen saturation can complicate the course whether or not sepsis is present.20,21 Follow standard community-acquired pneumonia protocols for pneumonia treatment22 (see chapter 65, “Pneumonia and Pulmonary Infiltrates” for further discussion). All antibiotics cross the placenta. Select pregnancy risk category B agents for treatment.
AIRWAY IN PREGNANCY
Fluid retention causes edema within the structures of the upper airway,23,24 and weight gain with adipose deposition can contribute to landmark distortion. Anticipate difficulty with mask ventilation, laryngoscopy, glottic visualization, and endotracheal intubation. Desaturation occurs quickly as a result of maternal increased oxygen consumption and decreased functional residual capacity.
During pregnancy, the incidence of Mallampati Class III airways increases (only the soft palate and base of the uvula are seen when the mouth is open and the tongue is protruding), making intubation more difficult (see Figure 29-8 in chapter titled “Intubation and Mechanical Ventilation”). Increased Mallampati scores correlate with gains in body weight,25 and because obesity in pregnancy is more common than in the nonpregnant state, this contributes to the likelihood of a difficult airway.26
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree