Respiratory Diseases of the Newborn
Craig H. Raskind
OVERVIEW
Respiratory diseases in the neonate often present as respiratory distress. Respiratory distress is a clinical mosaic composed of a combination of signs and symptoms that include elements of tachypnea, intercostal, subcostal and suprasternal retractions, nasal flaring, grunting, and cyanosis. It is one of most common reasons for admissions to the neonatal intensive care unit (NICU). The most common causes for NICU admission include transient tachypnea of the newborn (TTN) and infection (i.e., sepsis, pneumonia). Respiratory distress as a diagnostic sign has an expansive differential that includes multiple disease etiologies. These etiologies can be broadly divided into two classifications: nonrespiratory disorders and respiratory diseases.
Etiologies of nonrespiratory disorders causing respiratory distress are extensive and include:
Cardiovascular
Congenital heart disease
Congestive heart failure with secondary pulmonary edema
Hematologic
Severe anemia
Polycythemia/hyperviscosity syndrome
Metabolic
Metabolic acidosis
Hypoglycemia
Hypothermia
Neuromuscular
Central nervous system
Cerebral edema
Intracranial hemorrhage
Meningitis
Spinal muscular atrophy
Drug exposure
Peripheral nervous system
Myasthenia gravis
Muscular
Muscular dystrophy
Although it is essential and critical to consider cardiac disease at the outset of a neonatal evaluation, it is an uncommon cause of symptomatology immediately following birth.
Respiratory diseases causing respiratory distress during the neonatal period may be divided into four general categories:
1. Parenchymal conditions
2. Developmental abnormalities
3. Mechanical abnormalities
4. Airway abnormalities
Parenchymal conditions include transient tachypnea of the newborn (TTN), respiratory distress syndrome (RDS), bacterial pneumonia, meconium aspiration syndrome (MAS), and persistent pulmonary hypertension of the newborn (PPHN). Developmental abnormalities include congenital diaphragmatic hernia (CDH), congenital cystic adenomatoid malformation (CCAM), pulmonary sequestration, tracheoesophageal fistula (TEF), pulmonary hypoplasia, and infantile lobar emphysema. Mechanical abnormalities include all forms of pulmonary air-leak syndromes. Airway abnormalities may include any intrinsic or extrinsic causes leading to airway obstruction. Further elaboration and discussion on many of these conditions follow the questions listed in the following.
REVIEW EXERCISES
QUESTIONS
1. Embryologically the lung is derived from:
a) Lateral folds of embryonic mesoderm
b) Medial pharyngeal groove of the foregut endoderm
c) Epithelial cells of the neural crest
d) Subdivision of primordial mesenchyme
e) None of the above
View Answer
Answer
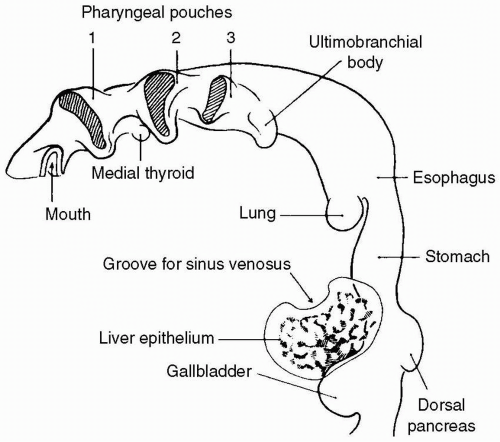
The answer is b. Lung development begins during the third week of gestation. It is during the embryonic period of fetal development that the lung bud differentiates from a ventral outpouching from the floor of the primitive foregut endoderm (Fig. 21.1).
Lung organogenesis can be divided into five distinct periods:
Embryonic (weeks 3-6)
Pseudoglandular (weeks 6-16)
Canalicular (weeks 16-26)
Saccular (weeks 26-36)
Alveolar (weeks 36-maturity)
During the embryonic and pseudoglandular periods the major conducting airways are established and elaborated upon through branching morphogenesis. The canalicular and saccular periods include vascularization of terminal respiratory units to form the adult respiratory unit and cytodifferentiation of bronchiolar and alveolar cells leading to the appearance of Type I and Type II surfactant-producing pneumocytes. Finally, it is during the alveolar period that a marked reduction of interstitial tissue and maturation of alveolar organization (alveolarization) occur. Subsequent alveolar development and lung growth, including further subdivision and proliferation of alveoli, continue well beyond infancy until approximately 2 to 6 years of age. As each stage of lung development is important for subsequent growth and maturation, defects in morphogenesis may be traced to aberrant or arrested development during these periods. For example, tracheoesophageal fistula and tracheal stenosis arise during the embryonic period. Congenital diaphragmatic hernia (CDH), congenital cystic adenomatoid malformation (CCAM), and bronchogenic cysts arise during the pseudoglandular period.
2. A term male newborn is delivered by repeat caesarean section (C/S). Rupture of membranes occurs at delivery. APGARs are 9 at 1 minute and 9 at 5 minutes. At 15 minutes of life the infant is noted to have tachypnea and mild intercostal retractions. He is acyanotic with peripheral oxygen saturation at 88% to 90% on room air. There is no cardiac murmur. Chest radiograph shows expansion of the lungs to 8 to 10 anterior ribs, perihilar streaking, and a fluid density in the right horizontal fissure. Of the following, the best therapy for this infant is:
a) Intravenous furosemide
b) Intravenous ampicillin and gentamicin
c) Tracheal intubation and surfactant
d) Intravenous prostaglandin E1
e) Supplemental oxygen by nasal cannula
View Answer
Answer
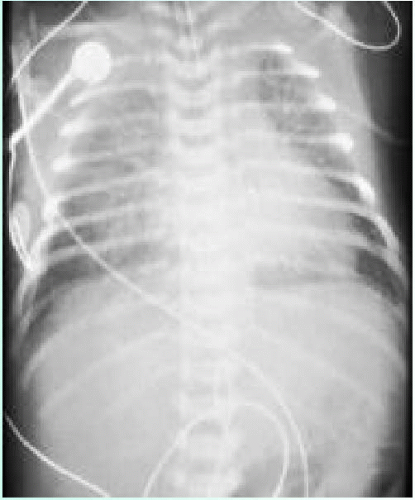
The answer is e. This infant most likely had transient tachypnea of the newborn (TTN), which is the most common cause of respiratory distress in newborns. It affects both term and preterm neonates. It results from the delayed clearance of fetal lung fluid after birth. This delayed clearance results from a combination of factors, including inhibition of apical Na+ channels activation through which lung fluid is reabsorbed, lack of inhibition of chloride-mediated lung fluid secretion, and absent or decreased mechanical forces associated with labor that are either absent with cesarean delivery or decreased with precipitous vaginal delivery. Symptoms may include a combination of grunting, nasal flaring, intercostal, subcostal, or suprasternal retractions, and tachypnea, with or without cyanosis. Resolution typically occurs by 48 to 72 hours after birth. This condition is usually benign and self-limiting, although not universally. Risk factors for TTN include:
Cesarean delivery without a trial of labor
Late-preterm delivery
Maternal diabetes
Precipitous delivery
Fetal distress
Maternal sedation
Perinatal depression
Roentgenographic evaluation (Fig. 21.2) generally demonstrates good lung volumes, increased interstitial markings, perihilar streaking without air bronchograms, fluid in fissure(s), and only occasionally pleural effusion. Arterial blood gas testing may indicate mild hypoxemia with or without hypercarbia. The differential diagnosis includes RDS, pneumonia, air leak syndromes, and congenital lung malformations. Treatment is supportive and may include oxygen supplementation, continuous positive airway pressure (CPAP), intravenous fluid administration, and close monitoring.
3. A 1600 g infant is born at 32 weeks gestation. The pregnancy was complicated by preterm labor and precipitous delivery. The mother received no antenatal steroids and her membranes ruptured just prior to delivery. At 1 to 2 hours of age, the infant develops tachypnea, grunting, nasal flaring, and subcostal retractions. Of the following, the most likely radiographic finding in this infant would be:
a) Fine reticulogranular pattern
b) Diffuse coarse infiltrates
c) Pleural effusion
d) Raised thymic silhouette (sail sign)
e) Fluid in the fissures
View Answer
Answer
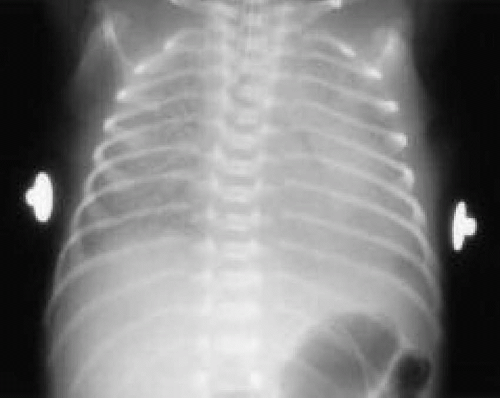
The answer is a. Respiratory distress syndrome (RDS) is the most common cause of severe respiratory distress in the newborn. It is a disease of both biological and biochemical immaturity, characterized by pulmonary surfactant deficiency. This deficiency leads to alveolar collapse at low lung volumes. The resultant atelectasis leads to ventilation-perfusion mismatching within the lung and pulmonary edema. These changes in turn lead to decreased lung compliance and altered gas exchange patterns, including hypoxemia, hypercarbia, and acidosis. The incidence of RDS is inversely proportional to gestational age, affecting nearly 100% of neonates delivered between 23 and 25 weeks of gestation, approximately 60% among neonates delivered by 29 weeks of gestation, 20% to 30% among those delivered at 29 to 34 weeks gestation, nearly 5% among those delivered at 34 to 37 weeks, and <1% among those delivered at >37 weeks. Onset of symptoms may be evident immediately after birth, or may develop or worsen within minutes to hours after birth and present with symptoms of respiratory distress. Symptoms may include tachypnea, grunting, nasal flaring, suprasternal, subcostal and intercostal retractions, and cyanosis. Roentgenographic evaluation (Fig. 21.3) demonstrates a characteristic fine reticulogranular pattern (“ground-glass” appearance) with air-bronchograms indicative of diffuse atelectasis. Peak severity occurs at 72 to 96 hours after birth. Recovery usually coincides with brisk urinary diuresis. Preventive strategies include maternal treatment with tocolytic agents to arrest premature labor and maternal antenatal corticosteroid therapy to accelerate fetal lung
maturity. Efficacy of the latter is best when administered 24 to 48 hours prior to delivery. When delivery occurs 7 to 14 days after maternal dosing, efficacy is limited, if at all efficacious. Complications associated with RDS include the development of air-leak syndromes (20%-50%) and increased incidence of chronic lung disease. Medical management of RDS may necessitate where indicated use of oxygen therapy, mechanical respiratory support, intravenous fluid and electrolyte therapy, and intratracheal surfactant replacement therapy. The use of exogenous surfactant replacement therapy is associated with decreased mortality, frequency of associated air-leak syndromes, and duration of mechanical ventilation. It has been demonstrated to reverse atelectasis and improve pulmonary functional residual capacity with a resultant improvement in gas exchange patterns, including oxygenation. Surfactant is commonly administered prophylactically to patients less than 29 weeks gestation at high risk for RDS and as rescue treatment in established moderate to severe RDS disease. The differential diagnosis includes TTN, pneumonia, and pleural effusion.
maturity. Efficacy of the latter is best when administered 24 to 48 hours prior to delivery. When delivery occurs 7 to 14 days after maternal dosing, efficacy is limited, if at all efficacious. Complications associated with RDS include the development of air-leak syndromes (20%-50%) and increased incidence of chronic lung disease. Medical management of RDS may necessitate where indicated use of oxygen therapy, mechanical respiratory support, intravenous fluid and electrolyte therapy, and intratracheal surfactant replacement therapy. The use of exogenous surfactant replacement therapy is associated with decreased mortality, frequency of associated air-leak syndromes, and duration of mechanical ventilation. It has been demonstrated to reverse atelectasis and improve pulmonary functional residual capacity with a resultant improvement in gas exchange patterns, including oxygenation. Surfactant is commonly administered prophylactically to patients less than 29 weeks gestation at high risk for RDS and as rescue treatment in established moderate to severe RDS disease. The differential diagnosis includes TTN, pneumonia, and pleural effusion.
4. A pregnant woman is counseled to have a repeat cesarean section as her pregnancy approaches term. To avoid delivering a newborn who has immature lungs and consequent respiratory distress syndrome, she undergoes antenatal assessment of her amniotic fluid. Of the following, the amniotic fluid test most likely to predict fetal lung maturity is:
a) Phosphatidylglycerol
b) Alpha-fetoprotein
c) Surfactant protein A
d) Thyroxine
e) Sphingomyelin
View Answer
Answer
The answer is a. Produced by type II pneumocytes, pulmonary surfactant is a complex mixture of both phospholipids and associated proteins. Its presence reduces alveolar surface tension, thereby helping maintain alveolar stability at low lung volumes (i.e., prevention of alveolar collapse at the end of expiration). This in turn prevents atelectasis while promoting efficient ventilation and oxygenation. Of its many components (Fig. 21.4), phosphatidylglycerol (PG) is a late-appearing surfactant component; when present in the amniotic fluid, the risk of developing RDS is <0.5%. Another biochemical marker useful in predicting lung maturation and adequacy of lung function at birth is the lecithin:sphingomyelin ratio (L:S ratio). Both lecithin (also known as phosphatidylcholine) and sphingomyelin are phospholipids found in the amniotic fluid. Although sphingomyelin remains at constant levels throughout gestation, lecithin, an active component of surfactant, is found in increasing amounts throughout gestation. The L:S ratio increases from approximately (1:1) at 31 to 32 weeks gestation to (2:1) by 35 weeks gestation. A ratio of greater than (2:1) indicates fetal lung maturity among nearly all nondiabetic pregnancies. For infants born to diabetic mothers (IDMs), an L:S ratio of 2.5:1 or greater is preferred for declaring fetal lung maturity.
5. The treatment of respiratory distress syndrome (RDS) with exogenous administration of surfactant is most likely to increase the incidence of:
a) Intraventricular hemorrhage
b) Bronchopulmonary dysplasia
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree