Respiratory
8-1 Asthma
Robert Hendrickson
Clinical Presentation
Patients with mild asthma exacerbations may present with cough, dyspnea, or pleuritic chest pain. Less commonly, patients present in extremis with symptoms developing from hypercarbia and hypoxemia, including altered mental status, anxiety, and respiratory arrest.
Physical examination findings vary with the severity of hypoxemia and airway obstruction. Mild signs may include tachycardia, tachypnea, and expiratory wheezing. As airway obstruction worsens, wheezing may be evident during both inspiration and expiration. With severe obstruction, air movement through the bronchi may be too decreased to detect wheezing at all. In these severe cases, pulsus paradoxus may occasionally be seen.1
Pathophysiology
Asthma is a reversible pulmonary obstructive airway disorder caused by bronchoconstriction, pulmonary mucosal edema, and mucosal hypersecretion.
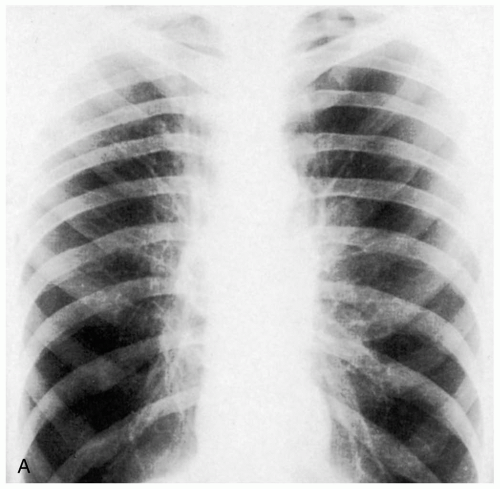
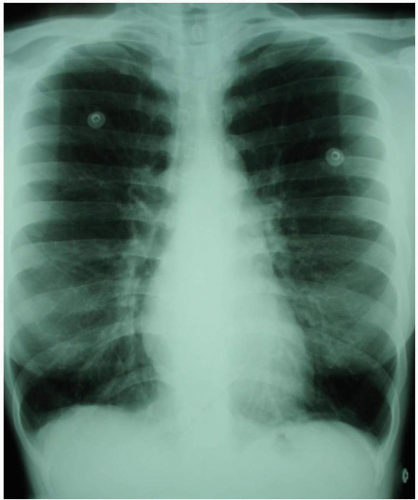 FIGURE 8-1 Radiographs of an adult with asthma, demonstrating hyperinflated lungs. (Courtesy of Anthony Morocco, MD.) |
Asthma exacerbations usually are triggered by viral infections, exposure to antigens (e.g., dog, cat, pollen), changes in weather (e.g., cold, humid), or exposure to irritants (e.g., ammonia, perfume). Other, less common triggers include gastroesophageal reflux, exercise, anxiety, nonsteroidal antiinflammatory drugs (NSAIDs), and menses.2
Asthmatic patients have an increased response to triggers compared with nonasthmatics. When an asthmatic encounters a trigger, the bronchiolar smooth muscle contracts, lessening the diameter of the airway. In addition, several inflammatory mediators, including histamine and leukotrienes, are released and cause mucosal edema and increased mucous secretion, which further decreases the airway lumen. Exposure to triggers may also increase vagal tone, particularly in children, causing bronchoconstriction.
Diagnosis
The diagnosis of mild asthma is made clinically from the typical history and physical examination. Occasionally, peak flow testing diagnoses an atypical asthma exacerbation, such as cough-variant asthma. In moderate or severe cases, pulse oximetry and peak flow testing may help predict the need for admission and help track improvements with therapy.3
Blood tests are of little value in the diagnosis. Chest radiography may reveal flattening of the diaphragm that is consistent with air trapping. In addition, chest radiography may be used to rule out pneumonia, heart failure, or pneumothorax, which sometimes mimic asthma. Arterial blood gas (ABG) analysis may be helpful to diagnose hypercarbia if the patient has an alteration of mental status, but it is not necessary to obtain as a “baseline” measurement.1 ABG results should not be the primary reason for deciding to initiate mechanical ventilation. Intubation and mechanical ventilation do little to improve the pulmonary dynamics of asthma and may increase the risk of barotrauma and air trapping. The decision to intubate should be based on the need to protect the airway (secondary to alterations of mental status, hypercarbia, and so on) or to improve severe hypoxemia.
Clinical Complications
Severe hypoxemia may result in myocardial infarction, cerebrovascular accident, hypoxic encephalopathy, respiratory failure, and death. Positive-pressure ventilation of an asthmatic patient may lead to hyperinflation, selfcontrolled positive end-expiratory pressure (auto-PEEP), hypotension, and barotrauma.
Management
An initial assessment of the need for an emergency airway and the adequacy of breathing should be rapidly performed, because it leads the initial management. The treatment of asthma is aimed at relieving bronchospasm and decreasing bronchiolar edema.
Patients in extremis in whom inhalational therapy is not practical (e.g., altered mental status, weak respiratory excursion) may receive subcutaneous β-adrenergic agonists or an intravenous terbutaline infusion in addition to inhaled/nebulized β-agonists. In addition, systemic corticosteroids and oxygen should be provided. Patients with mild to moderate asthma exacerbations may receive nebulized or inhaled β-agonists (albuterol or terbutaline) repeated hourly or given as a continuous infusion. The addition of an anticholinergic (vagolytic) inhaled agent (e.g., ipratropium bromide) may improve bronchodilatation.
Patients with moderate or severe asthma may be treated with corticosteroids, such a prednisone, 1 mg/kg orally; dexamethasone, 0.1 mg/kg intravenously; or Solu-Medrol (methylprednisolone) 2 mg/kg IV. Patients with an inflammatory condition as a trigger for their asthma (e.g., upper respiratory tract infection) should be treated with corticosteroids. The methylxanthines, theophylline and aminophylline, are still used occasionally to treat chronic asthma, but they have little role in acute asthma therapy. Magnesium has been used to treat acute asthma exacerbation with mixed results and remains controversial.
Patients with a complete response to therapy (peak expiratory flow [PEF] greater than 70%), may be discharged with a short course of oral corticosteroids and β-agonist therapy. Patients with incomplete response to therapy (PEF 50% to 70% with continued mild wheezing) may require admission to the hospital or discharge with close follow-up. Nonresponders (PEF less than 50%) require further therapy and hospitalization.
REFERENCES
1. Marik PE, Varon J, Fromm R. The management of acute severe asthma. J Emerg Med 2002;23:257-268.
2. Lemanske RF, Busse WW. Asthma. JAMA 1997;278:1855-1873.
3. Emond SD, Camargo CA, Nowak RM. 1997 National Asthma Education and Prevention Program guidelines: a practical summary for emergency physicians. Ann Emerg Med 1998;31:579-589.
8-2 Chronic Obstructive Pulmonary Disease
Jennifer Larson
Clinical Presentation
Patients with chronic obstructive pulmonary disease (COPD) may present with acute exacerbations beyond their baseline chronic airway obstruction and may have increased cough, sputum production, or dyspnea.1 Precipitating factors include infection (viral or bacterial) and bronchospasm.
Pathophysiology
Diagnosis
The diagnosis is based on the history and physical examination. Obstruction may be confirmed with peak flow and pulmonary function testing. On physical examination, there may be hyperresonance of the chest with wheezing and coarse rhonchi, or a decrease in air movement and lung sounds. Patients may exhibit accessory muscle use, pursed lips, or the tripod sitting position. Radiography may reveal hyperinflation with diminished pulmonary vasculature (emphysema) or increased bronchovascular markings (chronic bronchitis).1,2
Clinical Complications
Management
Patients with hypoxemia may be treated with supplemental oxygen to keep the blood oxygen saturation higher than 88% to 90%. Overaggressive oxygen therapy may lead to elevations in the carbon dioxide concentration, with subsequent narcosis and need for intubation. Patients with depressed mental status may require endotracheal intubation. Arterial blood gas (ABG) analysis helps to determine the extent of respiratory acidosis (pH less than 7.30 or carbon dioxide tension [PCO2] higher than baseline), and peak flow measurements determine the degree of airway obstruction. Emergency department (ED) management includes inhaled β-agonists and anticholinergics, corticosteroids, antibiotics (if bacterial infection appears to be the exacerbating factor), continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP), and intubation for respiratory failure. Patients who return to their baseline status with immediate therapies in the ED may be discharged home; however, they most likely will require a short steroid burst and close follow-up with their primary physician. Patients with poor responses to therapy require admission.1,2
REFERENCES
1. Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest 2002;121[5 Suppl]:121S-126S.
2. Singh JM, Palda VA, Stanbrook MB, Chapman KR. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease: a systematic review. Arch Intern Med 2002;162:2527-2536.
8-3 Pulmonary Embolism
Michael Greenberg
Clinical Presentation
The classic presentation of pulmonary embolism (PE) involves dyspnea, pleuritic chest pain, tachypnea, and tachycardia of relatively acute onset. However, patients may present with a wide variety of symptoms, ranging from asymptomatic or subtle to cardiorespiratory arrest. Patients with risk factors for or clinical evidence of venous thrombosis are at special risk.1,2,3
Pathophysiology
As many as 600,000 cases of PE occur annually in the United States, with up to 200,000 fatal events each year.1 Risk factors for venous thromboembolism include age greater than 40 years, previous history of venous thromboembolism, surgery with more than 30 minutes of anesthesia, prolonged immobilization, congestive heart failure (CHF), malignancy, lower-extremity and pelvic fractures, obesity, pregnancy or recent delivery, estrogencontaining medications, inflammatory bowel disease, and various genetic disorders.1
Diagnosis
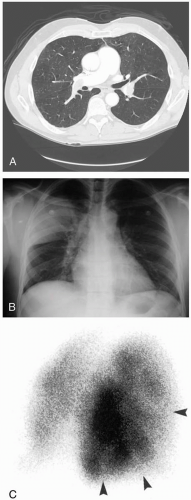
The diagnosis of PE is “confounded by a clinical presentation that may be subtle, atypical, or obscured by another coexisting disease.”1 Multiple and varied diagnostic tests exist, allowing assessments of probability for PE as “low” (10% or less), “intermediate” (about 30%), or “high” (70% or greater).1 Diagnostic tests that may be used include D-dimer testing, ventilation-perfusion scanning, computed tomography (CT), and pulmonary angiography. Pulmonary angiography remains the gold standard for diagnosing PE.1 The physical examination in conjunction with the electrocardiogram (ECG), chest radiograph, chest CT, and echocardiogram may show evidence of dysfunction of the right ventricle, which constitutes an important indicator of high risk as well as poor outcome.1,2 Measurements of troponin levels, pro-B-type natriuretic peptide, and B-type natriuretic peptide may also assist in evaluating right ventricular function. The ECG may show the classic “S1Q3T3” pattern.
Clinical Complications
Complications include ventricular dysfunction, respiratory failure, multiorgan failure, and death.
Management
Heparin anticoagulation is the mainstay of immediate therapy, with the initiation of longer-term anticoagulation as an essential component of care.1 Vena cava filters may be considered to reduce the chance of additional emboli getting to the lungs. Thrombolysis may be considered for use in some cases but is currently controversial. Surgical or catheter-assisted embolectomy may be considered for selected patients.1
REFERENCES
1. Fedullo PF, Tapson VF. Clinical practice: the evaluation of suspected pulmonary embolism. N Engl J Med 2003;349:1247-1256.
2. Goldhaber SZ, Elliott CG. Acute pulmonary embolism. Part II: risk stratification, treatment, and prevention. Circulation 2003;108:2834-2838.
3. Guidelines on diagnosis and management of acute pulmonary embolism. Task Force on Pulmonary Embolism, European Society of Cardiology. Eur Heart J 2000;21:1301-1336.
8-4 Spontaneous Pneumothorax
Swapan Dubey
Clinical Presentation
Patients with spontaneous pneumothorax (SP) usually present with acute pleuritic chest pain, dyspnea (proportional to the size of the pneumothorax), and cough. With a small pneumothorax, signs may include decreased breath sounds, decreased tactile fremitus, and ipsilateral hyperresonance. In the case of a large spontaneous pneumothorax, tachycardia, tachypnea, and hypoxemia may be evident.
Pathophysiology
Primary SP occurs without identifiable external precipitating factors in patients without obvious underlying lung disease. Secondary SP occurs as a complication of an underlying lung disease.1 In primary SP, disruption of the alveolar-pleural barrier is thought to occur when a subpleural bulla (or bleb), typically located at the lung apex, ruptures into the pleural space.1 Factors associated with primary SP include cigarette smoking and changes in ambient atmospheric pressure. Physical exertion does not appear to be a precipitating factor, whereas familial patterns, mitral valve prolapse, and Marfan’s syndrome are associated with SP.
Catamenial pneumothorax is a rare condition in which recurrent SPs occur in association with menses (typically within 72 hours of onset). Although it has been termed thoracic endometriosis syndrome, the exact cause of catamenial pneumothorax remains uncertain. One possibility is that this condition results from passage of intraabdominal air through diaphragmatic defects.1
Diagnosis
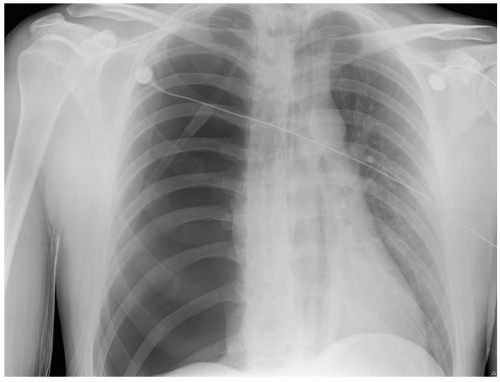
Suspicion based on the clinical presentation and physical examination are crucial in identifying SP. Chest radiography may reveal a visceral pleural line with absence of lung markings distal to the line. Formulas used to estimate size are unreliable. Associated electrocardiogram (ECG) changes may include axis deviation, decreased QRS voltage, and T-wave inversions.1
Clinical Complications
Approximately 3% of SPs become tension pneumothoraces, with possible associated hypoxemia, cyanosis, hypotension, and death. Reinflation pulmonary edema has been reported after correction of large SPs.1
Management
Supportive measures that should be initiated include intravenous (IV) access and supplemental oxygen. Treatment with oxygen by a nonrebreather mask may increase the rate of absorption of the pneumothorax. The ultimate goal of the management of an SP is evacuating the pleural space and minimizing the possibility of recurrence.1 Decisions in the management of a pneumothorax must take into account several factors, including size of the pneumothorax, severity of signs, presence of underlying pulmonary disease, comorbidities, patient reliability, and availability for follow-up monitoring. Needle aspiration of pneumothorax has been advocated by some, with a variable degree of success. Tube thoracostomy is the most common approach to the treatment of pneumothorax.1
REFERENCES
1. Weissberg D, Rafaely Y. Pneumothorax: experience with 1,199 patients. Chest 2000;117:1279-1285.
8-5 Severe Acute Respiratory Syndrome
Michael Greenberg
Clinical Presentation
Severe acute respiratory syndrome (SARS) begins with a prodrome, often with fever, chills and rigors, headache, malaise, and myalgias. After 3 to 7 days, a lower respiratory tract illness develops, in most cases with a dry, nonproductive cough or dyspnea, sometimes with hypoxemia. Diarrhea has been described in a large percentage of SARS patients.1,2,3,4
Pathophysiology
SARS is an emerging infectious disease that was first identified in China in November, 2002. It is caused by a unique coronavirus, SARS-CoV. The SARS-CoV genome has been sequenced, and it does not appear to be related to any previously known coronaviruses of humans or animals.2 It is likely that SARS-CoV started out as an animal virus that transformed to be able to accommodate human-to-human transmission. Within the first 10 days, the histology reflects diffuse alveolar damage with a mixture of inflammatory cells, edema, and hyaline membrane formation. Desquamation of pneumatocytes is a consistent finding.2
Diagnosis
The diagnosis requires an elevated index of suspicion in conjunction with findings on examination. Chest radiographs demonstrate either focal or generalized infiltrates in most cases, with no cavitation, lymphadenopathy, or pleural effusion.4 Lymphopenia, thrombocytopenia, and elevated lactate dehydrogenase (LDH) levels are common. The diagnosis may be confirmed by detection of serum antibody to SARS-CoV by enzyme-linked immunosorbent assay (ELISA), polymerase chain reaction (PCR), or other techniques. The differential diagnosis includes adult respiratory distress syndrome (ARDS), cytomegalovirus pneumonitis, acute interstitial pneumonitis, bronchiolitis obliterans-organizing pneumonia (BOOP), and alveolar proteinosis.
Clinical Complications
Management
In many cases, management requires admission to an intensive care unit (ICU) with mechanical ventilatory support. Some have advocated the use of ribavirin and corticosteroids. However, a number of cases have progressed to irreversibility despite all efforts. The optimal treatment for SARS has yet to be derived.
REFERENCES
1. Hui D, Wong P, Wang C. SARS: Clinical features and diagnosis. Respirology 2003;8[Suppl]:S20-S24.
2. Nicholls J, Dong X, Jiang, G, et al. SARS: Clinical virology and pathogenesis. Respirology 2003;8[Suppl]:S6-S8.
3. Yeung M, Xu R. SARS: epidemiology. Respirology 2003;8[Suppl]:S9-S14.
4. OOI G, Daqing M. SARS: radiological features. Respirology 2003;8[Suppl]:S15-S19.
8-6 Hospital-Acquired Pneumonia
Robert Hendrickson
Hospital-acquired pneumonia (HAP) is defined as a pulmonary infection that develops at least 48 hours after admission to the hospital. The emergency physician may see HAP in patients who recently were discharged from a health care facility, particularly an intensive care unit (ICU).1,2,3
Clinical Presentation
Patients may present to the emergency department (ED) with fever, cough, pleuritic chest pain, or some combination of these. Fever and leukocytosis, with or without a leftward differential shift, may be evident but are nonspecific and not essential to the diagnosis.1
Pathophysiology
HAP differs from community-acquired pneumonia (CAP) in that the pathogens that produce the disease are variable. In addition to the more typical staphylococcal and streptococcal infections, HAP may be produced by enteric gram-negative bacilli or by resistant organisms (e.g., Klebsiella, Pseudomonas, Acinetobacter).1 These organisms are present in hospitals and are thought to gain access to the pulmonary tree by microaspiration.
Diagnosis
The diagnosis of HAP is made in patients with respiratory symptoms and a pulmonary infiltrate who recently were discharged from a health care facility. It is important to consider alternative causes of pulmonary infiltrate in these patients, including pulmonary embolus and pulmonary edema.2 The reliability of other methods (e.g., computed tomography [CT], invasive microbiologic techniques) used to diagnose pneumonia in patients with clinical HAP is questionable.2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree